Abstract
Stem cell leukemia/T cell acute leukemia 1 (SCL/TAL1) plays a key role in the development of murine primitive hematopoiesis but its functions in adult definitive hematopoiesis are still unclear. Using lentiviral delivery of TAL1-directed shRNA in human hematopoietic cells, we show that decreased expression of TAL1 induced major disorders at different levels of adult hematopoietic cell development. Erythroid and myeloid cell production in cultures was dramatically decreased in TAL1-directed shRNA-expressing cells, whereas lymphoid B-cell development was normal. These results confirm the role of TAL1 in the erythroid compartment and show TLA1's implication in the function of myeloid committed progenitors. Moreover, long-term cultures and transplantation of TAL1-directed shRNA-expressing CD34+ cells into irradiated nonobese diabetic–severe combined immunodeficient (NOD-SCID) mice led to dramatically low levels of human cells of all lineages including the B-lymphoid lineage, strongly suggesting that TAL1 has a role in the early commitment of hematopoietic stem cells (HSCs) in humans. Cultures and transplantation experiments performed with mouse Sca1+ cells gave identical results. Altogether, these observations definitively show that TAL1 participates in the regulation of hematopoiesis from HSCs to myeloid progenitors, and pinpoint TAL1 as a master protein of human and murine adult hematopoiesis.
Introduction
Stem cell leukemia/T cell acute leukemia 1 (SCL/TAL1) belongs to a group of nuclear factors called basic helix-loop-helix (bHLH) transcription factors.1 During hematopoiesis, TAL1 is expressed mainly in erythroid cells, megakaryocytic cells, and hematopoietic stem cells (HSCs)2,3 where it has been implicated in different steps of development and differentiation.1 TAL1 can activate or repress transcription through TAL1 nuclear complexes containing transcriptional activators or repressors.4 These TAL1 nuclear transcription complexes are bound to lineage-specific regulatory regions in immature hematopoietic progenitors and undergo dynamic changes as HSCs differentiate, indicating the importance of TAL1 in the regulation of hematopoietic-specific genes from HSCs to differentiated hematopoietic progenitors.4-6
TAL1 is known to play a crucial role in the generation of blood cells during mouse embryonic development7,8 and mouse Tal1–/– embryonic stem cells fail to participate in hematopoiesis, even in a blastocyst environment,9 a failure that is rescued by re-expressing TAL1 before the onset of hematopoiesis.10 Whereas the role of TAL1 during adult erythropoiesis and megakaryopoiesis is well defined in humans and mice,11-14 its implication in the self-renewal and differentiation of adult HSCs and primitive hematopoietic progenitor cells is highly controversial. Conditional deletion of TAL1 in adult bone marrow (BM) cells reveals that TAL1 is either required15 or not13 to reconstitute hematopoiesis of lethally irradiated mice and, when it is required, the exact TAL1-dependent stem cell population is not clearly defined. Indeed, whereas deletion of TAL1 in mouse seems to alter short-term (ST) repopulating cells,15 enforced TAL1 expression improves the reconstitution ability of human long-term SCID-repopulating cells (LT-SRCs), possibly through their increased self-renewal.16 These observations partly correlate with the effects of enforced TAL1 expression in mouse HSCs in transgenic mice or using retroviral gene expression where differentiation of TAL1-overexpressing primitive cells showed increased myelopoiesis.17,18 However, in these mouse studies, the effect of enforced TAL1 expression on the HSC compartment was not investigated and remains to be explored.
To address the physiologic role of TAL1 in the HSC compartment and to identify TAL1-dependent progenitors, we have developed an shRNA-based strategy that specifically targets human or mouse TAL1 transcripts. We show here, and for the first time in humans, that normal TAL1 expression is required at different levels of the hematopoietic hierarchy in mouse and humans. Indeed, TAL1 decreased expression interferes with the differentiation of erythroid and, unexpectedly, of granulocyte/macrophage (GM) committed progenitors, whereas B-lymphoid cell production is not affected. Low TAL1 expression also profoundly alters the ability of LT- and ST-HSCs and immature hematopoietic progenitors to reconstitute hematopoiesis. These data definitively prove that TAL1 is a major regulator of human and mouse hematopoietic development in adults.
Materials and methods
Lentiviral vectors
Sense and antisense oligonucleotides (19 nucleotides, sequences shown in Figure 1A) were designed in the bHLH coding region of the human TAL1 gene. They were used for polymerase chain reaction (PCR) amplification, and the DNA fragment obtained was cloned in the pSuper plasmid 3′ of the polIII H1promoter. H1-shRNATAL1 DNA fragment was then subcloned in the pTRIP/ΔU3-EF1α encoding the green fluorescent protein (TRIP/ΔU3-EF1α-GFP19 ) lentiviral vector. An shRNA directed against the human hepatitis B virus was used as control (ctrl).20 Production of both shRNA-TAL1 and shRNActrl lentiviral vectors was performed as described.12 A murine TRIP/ΔU3-MND-GFP was constructed to inhibit mouse TAL1 gene expression using oligonucleotides directed against mouse TAL1 transcripts (sequences shown in Figure 5A).21
CD34+ cells
Cord blood (CB) samples were collected from healthy infants with the informed consent of the mothers, according to approved institutional guidelines. CD34+ cells were purified by immunomagnetic selection (Stem Cell Technologies, Vancouver, BC, Canada). CD34+ cells (purity ≥ 85%) were used immediately or after storage in liquid nitrogen.
Murine Sca1+ cells
Sca1+ cells (purity ≥ 90%) were isolated from the BM of 8- to 10-week-old C57BL/6 mice housed in the animal colony of the Institut Cochin, Paris, France, by immunomagnetic selection using the EasySep kit (Stem Cell Technologies) and a rat anti–Sca1-PE antibody (cloneE13-167.7; Becton Dickinson/Pharmingen, Le Pont de Claix, France).
Transduction
CD34+ cells (106 cell/mL) were cultured for 72 hours in Iscove medium (IMDM; Invitrogen, Cergy-Pontoise, France) supplemented with 15% bovine serum albumin (BSA)/insulin/transferrin (BIT 9500; Stem Cell Technologies), with recombinant human (rhu) stem cell factor (SCF; 0.1 μg/mL; Amgen, Neuilly-sur-Seine, France), Flt3-ligand (FL; 0.1 μg/mL; Diaclone, Besancon, France), interleukin-3 (IL-3; 60 ng/mL; Diaclone), and TPO peptide (pTPO; 10 nM22 ). Concentrated shRNATAL1 or shRNActrl lentiviral vectors were added at a concentration of 2.5 μg/mL P24 twice at 24-hour intervals.23
Sca1+ cells (106 cells/mL) were cultured for 48 hours in IMDM/15% BIT in the presence of murine (m) IL-3 (20 ng/mL), mSCF (0.1 μg/mL) (both from Stem Cell Technologies), rhuIL-6 (10 ng/mL), pTPO (10 nM), and rhuFL (0.1 μg/mL). Concentrated vectors (2 μg/mL P24) were added twice at 24-hour intervals.
After transduction, the cells were washed twice and counted, and GFP expression was analyzed by fluorescence activated cell sorting (FACS).
Jurkat and MEL cells were transduced with 0.5 μg/mL and 1 μg/mL P24, respectively, of shRNA vectors for 48 hours, in α miminum essential medium (αMEM) containing 10% fetal calf serum (FCS; Invitrogen). Cells were then washed and assayed for TAL1 expression by Western blot.
Colony-forming units (CFUs)
Duplicates of 300 transduced CD34+ cells were seeded in methylcellulose containing 30% FCS (Gibco, Cergy Pontoise, France), 10% BSA (Stem Cell Technologies), 1% l-glutamine, 10–4 M beta-mercapto-ethanol, IL-3 (8 ng/mL), erythropoietin (2 IU/mL; Kirin Brewery, Gunma, Japan), SCF (50 ng/mL), granulocyte–colony-stimulating factor (G-CSF; 25 ng/mL; Amgen) and granulocyte macrophage (GM)–CSF (10 ng/mL; Schering Plough, Dardilly, France). After 14 days, the number of GFP+ burst-forming units of erythroid cells (BFU-e's) and GM-CFUs was determined using a fluorescence microscope (Nikon Eclipse TE2000-S; Nikon, Champigny-sur-Marne, France).
GFP+ CFUs derived from transduced Sca1+ mouse BM cells were identified using Methocult GF M3434 (Stem Cell Technologies).
Short-term liquid culture
Transduced CD34+ cells (105/mL) were cultured in IMDM/15% BIT containing IL-3 (10 ng/mL), SCF (10 ng/mL), FL (50 ng/mL), and G-CSF (15 ng/mL) for 15 days. Proliferation and myeloid (based on the CD15 expression marker) differentiation of GFP+ cells were analyzed.
Long-term liquid culture
CD34+ transduced cells (2500-5000) were cultured on a confluent layer of murine MS5 stromal cells in long-term culture (LTC; Stem Cell Technologies) medium.24 Cells were incubated at 33°C and fed every week. At 5 and 10 weeks later, the progeny of CD34+ cells was harvested, counted, and analyzed by FACS for the expression of GFP and cell surface markers. Cells were also plated in CFU assays.
Transplantations
NOD/LtSz-scid/scid mice were housed in the Institut André Lwoff, Villejuif, France. NOD/SCID and C57BL/6 mice were irradiated at 3.25 Gy and 9 Gy, respectively, and NOD-SCID mice received a dose of 250 μg anti-CD122 antibody to abrogate residual natural killer activity 24 hours before intravenous or intrabone injection of total transduced cells (an equivalent of 1 × 105 to 2 × 105 CD34+ or Sca1+ day 0 input cells). Mice were bled or killed and their hematopoietic organs analyzed for the presence of transduced human or mouse cells by FACS.16 In the case of intrabone injection, the injected marrow was analyzed separately from the 3 other bones.
All experiments and procedures were performed in compliance with French Ministry of Agriculture regulations for animal experimentation.
Flow cytometry
Lineage analysis of hematopoietic cells was performed on a FACSCalibur flow cytometer (Becton Dickinson). PE-, PC5-, or APC-conjugated mouse antihuman monoclonal antibodies (MoAbs) CD45 (clone J.33), CD34 (clone 581), CD14 (clone RM052), CD15 (clone 80H5), and CD19 (clone J4.119) (all from Beckman Coulter, Villepinte, France) and rat antimouse MoAbs CD3ϵ (clone 2C11), B220 (clone RA3-6B2), and CD11b/MAC1 (clone M1/70) (all from Pharmingen) were used.
Western blot
TAL1 protein expression was analyzed by Western blotting. Briefly, 5 × 105 transduced CD34+ CB, Jurkat, or mouse erythroleukemia (MEL) cells were lysed and analyzed as previously described12 using TAL1 antibodies generously provided by D. Matthieu (Institut de Génétique Moléculaire, Montpellier, France) and C. Porcher (University of Oxford, United Kingdom). Normalization by β-actin was performed using anti–β-actin antibody (Sigma Aldrich, Lyon, France).
Statistics
Statistical analysis was performed using the paired Student t test for results of cultures and the Mann-Whitney U test for mouse data.
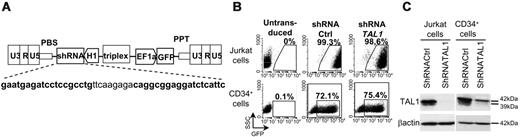
ShRNATAL1 lentiviral vector efficiently decreases TAL1 expression in hematopoietic cells. (A) Lentiviral construct for human shRNATAL1 and GFP expression. Bold characters correspond to sense and antisense oligonucleotides used for shRNATAL1 design. (B) Transduction efficiency assessed by GFP expression in Jurkat cells and CD34+ cells after shRNActrl and shRNATAL1 vector transduction. (C) Western blot analysis of human TAL1 protein in total unsorted transduced cells. β-actin is used as protein loading control.
ShRNATAL1 lentiviral vector efficiently decreases TAL1 expression in hematopoietic cells. (A) Lentiviral construct for human shRNATAL1 and GFP expression. Bold characters correspond to sense and antisense oligonucleotides used for shRNATAL1 design. (B) Transduction efficiency assessed by GFP expression in Jurkat cells and CD34+ cells after shRNActrl and shRNATAL1 vector transduction. (C) Western blot analysis of human TAL1 protein in total unsorted transduced cells. β-actin is used as protein loading control.
Results
Lentiviral-directed shRNA vector efficiently inhibits TAL1 expression
Jurkat and CD34+ cord blood (CB) cells were transduced for 3 days with TRIP/ΔU3-shRNA/EF1α-GFP lentiviral vectors (Figure 1A). The transduction efficiency was monitored by FACS analysis to follow GFP expression and the ability of the TAL1-directed shRNA to decrease TAL1 protein expression was determined by Western Blot analysis on transduced cell extracts. Jurkat cells were efficiently transduced since GFP was expressed by more than 98% of cells (Figure 1B) and shRNATAL1 induced a dramatic decrease of the expression of the 2 TAL1 major isoforms (Figure 1C). CD34+ cells were less efficiently transduced, with a proportion of GFP+ cells that reached up to 80% for shRNActrl (mean ± standard deviation [SD]: 69.7% ± 10.9%, n = 10) and shRNATAL1 (71.3% ± 7.3%, n = 10) constructs (Figure 1B). TAL1 expression in shRNATAL1 CD34+ cells was also significantly decreased compared with control cells (Figure 1C), allowing functional assays to be performed.
Decreased expression of TAL1 protein affects both committed myeloid and erythroid progenitors
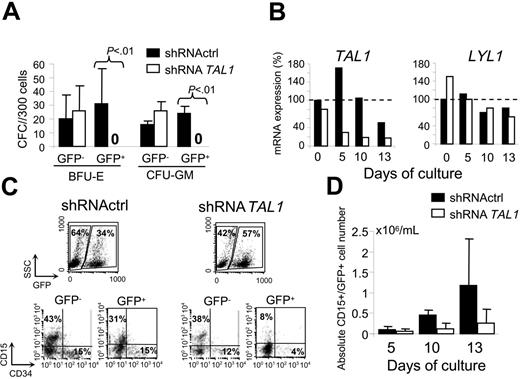
To investigate the role of TAL1 in committed progenitors, transduced CD34+ cells were first assayed for myeloid GM-CFU and erythroid BFU-E clonogenic progenitors. Decreased TAL1 expression led to a 2- to 3-fold reduction of the total number of erythroid (58.5 ± 42.9) versus BFU-E (17.4 ± 17.9) (P = .02) progenitors and of myeloid (34.9 ± 8.2) versus GM-CFU (18.8 ± 6.5) (P = .001) progenitors. The decrease of shRNATAL1-CD34+– derived CFUs was related to the lack of GFP+ colonies, whereas no significant difference was observed in colonies generated from GFP– fractions (Figure 2A). These results indicated that decreased expression of TAL1 protein leads to the loss of both erythroid and GM committed progenitors.
The absence of GM colonies strongly suggests for the first time the importance of TAL1 in the myeloid compartment and prompted us to study TAL1 expression during granulocytic differentiation. Reverse transcriptase (RT)–PCR analysis of sorted CD34+/GFP+ cells showed that, in control cells, TAL1 was expressed during early granulocytic differentiation, whereas its expression decreased thereafter (Figure 2B). As expected, TAL1 expression was decreased in shRNATAL1 cells in the granulocytic differentiation condition (Figure 2B), a result that correlated with an impairment of the production of shRNATAL1-derived granulocytic precursors (Figure 2C). Indeed, in the shRNATAL1 condition, a 4-fold reduction of granulocytic CD15+ cell production was detected at days 10 and 13 in the GFP+ fraction compared with the GFP– one or to both GFP+ and GFP– fractions of the control condition (Figure 2C-D). Interestingly, expression of LYL1 transcripts, a TAL1 close-related bHLH protein, did not vary in the shRNATAL1 cells during the culture period (Figure 2B).
Altogether, these results indicate that TAL1 is required for both GM and erythroid cell production from human committed progenitors.
TAL1 expression is required for efficient lymphoid and myeloid cell production from immature progenitors
We previously showed that the enforced expression of TAL1 enhances the self-renewal potential of human adult primitive hematopoietic cell populations.12,16 To study the effects of TAL1's decreased expression on immature hematopoietic progenitors, we used the long-term culture (LTC) of CD34+ cells on stromal cells since it provides information on immature progenitors by analysis of their progeny at different culture time points. Analysis of 5-week LTC showed that shRNATAL1 impaired the production of myeloid and erythroid progenitors from CD34+ cells as determined by the absence of CFUs (data not shown) and by the decrease in the proportion of CD14+/CD15+ cells (Figure 3A). Accordingly, decreased TAL1 expression led to a 3-fold decrease in the total number of myeloid CD14+/CD15+ cells (12 194 ± 4238 cells; n = 3) as compared with control CD34+ cells (42 662 ± 14 794 cells; n = 3). On the contrary, the production of CD19+ B lymphoid progenitors was not affected (Figure 3A-B), strengthening a role for TAL1 in progenitors already committed toward myelo/erythroid but not B-lymphoid lineage. However, when the LTC was carried over a 10-week period, both myeloid and B-lymphoid progeny were dramatically affected with, respectively, a 20-fold (17 963 ± 827 vs 924 ± 689 cells; n = 2) and 7-fold (27 820 ± 3068 vs 4014 ± 2426 cells; n = 2) decrease in their absolute numbers (Figure 3B, right panel). Thus, in addition to its role in the myeloid compartment, TAL1 also regulates a more primitive cell compartment, possibly multipotent. Furthermore, enforced expression of TAL1 in CD34+ cells transduced with the TRIP/ΔU3/EF1α-TAL1 lentiviral construct12 increased their myeloid cell production with 12.5 times more CD14+/CD15+ cells than in control cells (223 998 vs 17 963 cells; Figure 3B, right panel), whereas the absolute number of B-lymphoid CD19+ cells remained similar (Figure 3B). Altogether, these results strongly argue for a role of TAL1 on primitive lympho/myeloid progenitor cells thereafter restricted to the myelo/erythroid compartment.
TAL1 is required for the development of human myeloid committed progenitors. Transduced CD34+ cells were plated in methylcellulose culture and in myeloid differentiation in liquid culture. (A) GFP+ and GFP– colonies from BFU-Es and GM-CFU cells 14 days later. (B) TAL1 and LYL1 expression during myeloid differentiation detected by real-time quantitative PCR; dashed line indicates the arbitrary 100% of TAL1 expression determined in shRNActrl cells at day 0 of the culture. Shown is the mean of 2 measurements. (C) Analysis of CD15 and CD34 marker expression after 10 days of culture in both GFP– and GFP+ cells (one representative experiment, 10 days). (D) Evolution of GFP+/CD15+ cell numbers during the culture. Data are expressed as the mean plus or minus the standard error (SE) of 2 to 5 independent experiments.
TAL1 is required for the development of human myeloid committed progenitors. Transduced CD34+ cells were plated in methylcellulose culture and in myeloid differentiation in liquid culture. (A) GFP+ and GFP– colonies from BFU-Es and GM-CFU cells 14 days later. (B) TAL1 and LYL1 expression during myeloid differentiation detected by real-time quantitative PCR; dashed line indicates the arbitrary 100% of TAL1 expression determined in shRNActrl cells at day 0 of the culture. Shown is the mean of 2 measurements. (C) Analysis of CD15 and CD34 marker expression after 10 days of culture in both GFP– and GFP+ cells (one representative experiment, 10 days). (D) Evolution of GFP+/CD15+ cell numbers during the culture. Data are expressed as the mean plus or minus the standard error (SE) of 2 to 5 independent experiments.
Decreased TAL1 expression impairs myeloid and B-lymphoid cell development from immature progenitors. Transduced CD34+ cells were seeded in LTC on MS5 cells and the progeny of LTC-IC was analyzed 5 and 10 weeks later. (A) Percent of myeloid (CD14+/CD15+) and B-lymphoid (CD19+) cells were determined in GFP+ populations by FACS analysis in shRNActrl (left panels) and shRNATAL1 (right panels) conditions (one representative experiment, 10 weeks). (B) Absolute numbers of CD14++CD15+ and B CD19+ cells issued from shRNActrl and shRNATAL1 LTC-IC at 5 weeks (left panel) and 10 weeks (right panel) of coculture. The progeny of LTC-IC overexpressing TAL1 is also shown (10 weeks, right panel, one experiment). Data are expressed as the mean plus or minus SE of 3 independent experiments.
Decreased TAL1 expression impairs myeloid and B-lymphoid cell development from immature progenitors. Transduced CD34+ cells were seeded in LTC on MS5 cells and the progeny of LTC-IC was analyzed 5 and 10 weeks later. (A) Percent of myeloid (CD14+/CD15+) and B-lymphoid (CD19+) cells were determined in GFP+ populations by FACS analysis in shRNActrl (left panels) and shRNATAL1 (right panels) conditions (one representative experiment, 10 weeks). (B) Absolute numbers of CD14++CD15+ and B CD19+ cells issued from shRNActrl and shRNATAL1 LTC-IC at 5 weeks (left panel) and 10 weeks (right panel) of coculture. The progeny of LTC-IC overexpressing TAL1 is also shown (10 weeks, right panel, one experiment). Data are expressed as the mean plus or minus SE of 3 independent experiments.
Decreased TAL1 expression impairs human hematopoiesis in NOD-SCID mice
The results obtained on the LTC progenitors led us to investigate the effect of TAL1 decreased expression on the most immature progenitor cell compartment. The human HSC compartment was studied using transplantation of transduced CD34+ cells into NOD-SCID mice. Analysis of the mice 6 and 12 weeks after transplantation provides insights on short-term SRCs (ST-SRCs) and LT-SRCs, respectively.25,26 At both time points, reduced TAL1 expression in SRCs dramatically decreased the proportion of their CD45+/GFP+ progeny in the bone marrow compared with controls (Figure 4A). In all experiments the expression of shRNATAL1 lowered the levels of CD45+/GFP+ cells by 3- and 19-fold for ST- and LT-SRCs, respectively (Figure 4B). Similar results were obtained whatever the transplantation route used, that is, intravenously or directly in the mouse femur (Figure 4B, compare black and white dots). Analysis of the phenotype of the remaining GFP+ engrafted cells (possible for only a small number of mice) in the shRNATAL1 condition showed a trend toward a decreased proportion of myeloid cells although the ratio between myeloid (CD14+/ CD15+) and B-lymphoid (CD19+) CD45+/GFP+ cells was not statistically different compared with shRNActrl conditions (Figure 4C). These results indicated that the few (median values 3.9% and 1.1% for ST- and LT-SRCs, respectively) remaining GFP+ cells were derived from SRCs that did not express a sufficient level of shRNATAL1. Altogether, these results are consistent with a defect of low TAL1-expressing ST- and LT-SRCs in generating normal levels of hematopoiesis following transplantation, showing a role of TAL1 in human HSCs.
Decreased expression of TAL1 alters human hematopoietic recovery after transplantation into NOD-SCID mice. Transduced CD34+ cells were injected into NOD-SCID mice for SRC analysis. FACS analysis of mice bone marrow was performed 6 weeks and 12 weeks after transplantation. (A) Engraftment of human CD45+/GFP+ and human CD45+/GFP– cells in 2 representative mice 12 weeks after injection of shRNActrl or shRNATAL1 transduced CD34+ cells. (B) Engraftment levels of GFP+ cells for mice that underwent transplantation either intravenously (•) or intrabone (○); the horizontal bars indicate median % of CD45+/GFP+ cells measured for injected mice of each condition. (C) When the level of engraftment was sufficient, the relative proportion of myeloid CD14+/CD15+ and B-lymphoid CD19+ cells issued from ST-SRCs and LT-SRCs was determined. Lower and upper SE bars are related to CD14+/CD15+ and CD19+ cells, respectively.
Decreased expression of TAL1 alters human hematopoietic recovery after transplantation into NOD-SCID mice. Transduced CD34+ cells were injected into NOD-SCID mice for SRC analysis. FACS analysis of mice bone marrow was performed 6 weeks and 12 weeks after transplantation. (A) Engraftment of human CD45+/GFP+ and human CD45+/GFP– cells in 2 representative mice 12 weeks after injection of shRNActrl or shRNATAL1 transduced CD34+ cells. (B) Engraftment levels of GFP+ cells for mice that underwent transplantation either intravenously (•) or intrabone (○); the horizontal bars indicate median % of CD45+/GFP+ cells measured for injected mice of each condition. (C) When the level of engraftment was sufficient, the relative proportion of myeloid CD14+/CD15+ and B-lymphoid CD19+ cells issued from ST-SRCs and LT-SRCs was determined. Lower and upper SE bars are related to CD14+/CD15+ and CD19+ cells, respectively.
Decreased TAL1 expression impairs murine adult hematopoiesis
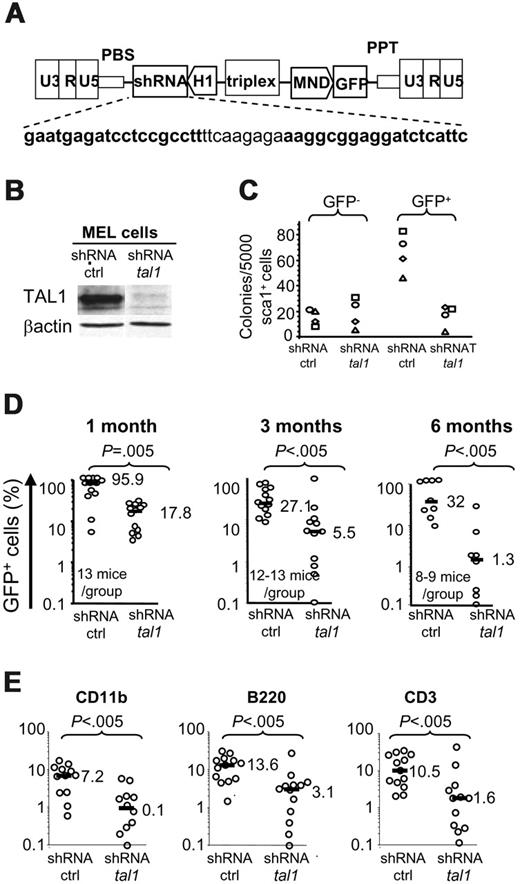
To determine whether decreased TAL1 expression also modified mouse hematopoiesis, cultures and transplantation experiments were performed using murine Sca1+ BM cells using a murine shRNAtal1 construct (Figure 5A). The murine shRNAtal1 vector almost completely abrogated the expression of TAL1 protein in erythroleukemic MEL cells (Figure 5B). ShRNATAL1 induced in vitro a decrease of all types of GFP+ CFUs produced from mouse Sca1+ cells when compared with the control vector (Figure 5C, right panel), whereas no difference was observed in the GFP– colonies in either condition (Figure 5C, left panel). These results indicated that differentiation of mouse myeloid committed progenitors requires the sustained expression of TAL1. Analysis of murine HSC progeny 1 to 6 months after transplantation showed a low contribution of shRNAtal1/HSC to the repopulation of lethally irradiated mice. Between 1 to 2 months, high levels (median: 89.5%-95.9%; n = 13 mice, 4 experiments) of GFP+ cells were detected in the peripheral blood of control mice, whereas up to an 18-fold reduction of GFP+ cells (median: 4.9%-17.8%; n = 9-13 mice, 4 experiments) was detected in the mice expressing shRNA-tal1 (Figure 5D and data not shown), indicating a role of TAL1 in the development of ST-HSCs. A lower proportion of GFP+ cells (only 30%) was observed in peripheral blood of control mice analyzed 3 and 6 months after transplantation, maybe due to a less efficient transduction of LT-HSCs (median: 27.1%, n = 13 mice, and 32%, n = 8-9 mice; 4 experiments). However, the percent of GFP+ cells remained 5 and 25 times lower in the mice expressing shRNAtal1 after 3 and 6 months, respectively (median: 5.5% and 1.3%; Figure 5D, middle and right panels) showing that TAL1 also regulated hematopoietic development from LT-HSCs. Importantly, all lymphoid, T and B, and myeloid lineages were decreased in mice that underwent transplantation with shRNAtal1/Sca1+ cells, indicative of an effect on the multipotential HSC compartment (Figure 5E). These results are in perfect concordance with those obtained with human cells, thus excluding a species-dependent effect and evidencing TAL1 as a key factor of adult hematopoiesis.
Decreased tal1 expression levels impair murine adult hematopoiesis. (A) Lentiviral construct for murine shRNAtal1 as in Figure 1. (B) Western blot analysis of TAL1 protein in shRNActrl and shRNAtal1 transduced MEL cells. (C) Transduced Sca1+ cells were plated in semisolid culture and the colonies issued from shRNActrl and shRNAtal1 transduced (GFP+) and nontransduced (GFP–) clonogenic progenitors were counted. Each symbol represents an independent experiment. (D) At 1, 3, and 6 months after Sca1+ cell transplantation, engraftment levels in the peripheral blood of treated mice were determined by the percentage of GFP+; the horizontal bars indicate the median % of GFP+ cells. (E) Percent of GFP+ myeloid (CD11b), B-lymphoid (B220) and T-lymphoid (CD3) cells in the blood of 3-month shRNActrl and shRNAtal1 mice.
Decreased tal1 expression levels impair murine adult hematopoiesis. (A) Lentiviral construct for murine shRNAtal1 as in Figure 1. (B) Western blot analysis of TAL1 protein in shRNActrl and shRNAtal1 transduced MEL cells. (C) Transduced Sca1+ cells were plated in semisolid culture and the colonies issued from shRNActrl and shRNAtal1 transduced (GFP+) and nontransduced (GFP–) clonogenic progenitors were counted. Each symbol represents an independent experiment. (D) At 1, 3, and 6 months after Sca1+ cell transplantation, engraftment levels in the peripheral blood of treated mice were determined by the percentage of GFP+; the horizontal bars indicate the median % of GFP+ cells. (E) Percent of GFP+ myeloid (CD11b), B-lymphoid (B220) and T-lymphoid (CD3) cells in the blood of 3-month shRNActrl and shRNAtal1 mice.
Discussion
The present study investigated the role of SCL/TAL1 in human and mouse adult hematopoiesis and focused on the effects of decreased TAL1 expression in the whole hierarchy of both human CD34+ cord blood and murine Sca1+ hematopoietic cells. The observations conclusively show that a low TAL1 expression level severely impairs both human and murine adult hematopoietic development.
As expected, low TAL1 expression resulted in decreased colony formation from CD34+ erythroid progenitors.12 Also, and at odds with previous reports describing opposing effects of TAL1 in erythroid versus myeloid cell differentiation,12,27-29 a dramatic decrease in numbers of shRNATAL1 human and mouse GM-CFU progenitors was found. These results are in agreement with the TAL1 expression detected in myeloid-restricted progenitors30 and with the increase of myeloid progenitors and mature cells obtained after TAL1-enforced expression in mouse hematopoietic progenitors.17,18 Moreover, they correlate with results obtained in zebra fish showing that morpholino-induced decreased TAL1 expression impairs myeloid cell development.31 Together with the defect in CD15+ and CD36+/GPA+ (data not shown) cell production in specific differentiating liquid cultures, our data strongly argue for the necessity of sustained TAL1 expression in erythroid and in myeloid committed progenitors.
Decreased TAL1 expression also resulted in low myeloid production in 5-week LTC where it abolished the detection of CFUs and strongly reduced the production of CD14+/CD15+ myeloid cells. We have previously showed that enforced TAL1 expression increased LTC-IC–derived CFU numbers, whereas a DNA binding domain-deleted mutant TAL1 did not significantly influence this compartment.12,16 Interestingly, low TAL1 expression did not affect B-lymphoid production in 5-week LTC, consistent with the absence of TAL1 expression in the B-cell lineage.30 This also argues against nonspecific effects of shRNA-TAL1 in human progenitors. Altogether, these observations indicate that TAL1 is required in myeloid-restricted progenitors. By contrast, analysis of the differentiating potential of immature progenitors by extending the LTC up to 10 weeks evidenced that both myeloid and B-lymphoid cell productions were decreased in the presence of shRNATAL1. Indirectly, this result suggested that extending LTC to 10 weeks reveals the progeny of a different class of progenitors compared with 5-week LTC, possibly multipotential lympho/myeloid progenitors.32,33 In these conditions, sustained TAL1 expression was required to maintain normal levels of both lymphoid and myeloid cell productions from these multipotent progenitors. In addition, in the 10-week LTC condition, enforced TAL1 expression increased the production of myeloid cells but not of CD19+ B cells. The lack of enforced TAL1 effect on the B-lymphoid compartment generated in the 10-week LTC is most probably the cumulative result of a positive effect on multipotential cells and the decreased B-cell differentiation. This result not only strengthened the involvement of TAL1 in the myeloid compartment but also showed subtle regulations according to the presence or absence of TAL1 expression, to the type and to the differentiation state of target hematopoietic cells. Indeed, it has previously been shown that enforced TAL1 expression decreases the production of B-lymphoid precursors probably related to bHLH class I E2A protein titration.16,18,34 In summary, TAL1 is required for the cell fate of lympho/myeloid progenitors and, when the myeloid and lymphoid specifications have occurred, TAL1 function is restricted to committed myeloid and erythroid progenitors but not to B-lymphoid progenitors. Whether TAL1 is required in B/myeloid progenitors where it has been shown to be expressed35 or whether TAL1 plays a role in more immature cells, also exhibiting T-cell potential,36 requires further investigation.
Evidence for a role of TAL1 in the hematopoietic primitive cell compartment comes from the analysis of repopulating abilities of human CB ST-SRCs and LT-SRCs and of mouse BM ST-HSCs and LT-HSCs. Indeed, transplantation experiments indicated that low TAL1 expression dramatically reduced all human and mouse HSC-derived progeny, indicating that TAL1 is a crucial regulator of the differentiation of HSCs, independent of species and cell sources. The injection of transduced CD34+ cells directly in the mouse BM did not rescue the human hematopoietic development defect, turning down a role of TAL1 in the migration of injected cells from peripheral blood to the BM. In fact, these results are identical to the ones we recently obtained using CB CD34+ cells expressing the DNA binding mutant ΔbTAL1.16 Indeed, when CB CD34+ cells expressed ΔbTAL1, NOD-SCID mice engraftment levels were extremely low and the few engrafted cells expressed only low levels of transgene.16 Taken together, all these results demonstrated the specificity of TAL1 effects on primitive cell compartments and the necessity of sustained levels of the entire protein for normal HSC activities. Our data are in agreement with those obtained by conditional Tal1 gene targeting (condtal1–/–), indicating a role for TAL1 in multipotent progenitors or ST-HSCs,15 but they contrast with those indicating that TAL1 was dispensable for HSC engraftment, self-renewal, and differentiation.13,15 Such discrepancies may be linked to the experimental procedures used in the different studies. In the present work, HSCs were used immediately after TAL1 decreased expression in classic in vitro and in vivo assays that are nonetheless very stressful for the cells. On the contrary, condtal1–/– HSCs were used several days or months after Tal1 gene deletion.13,15 Gene deletion strategy results in the total absence of Tal1 gene expression and may lead to a compensation mechanism—for instance, by other bHLH gene activation—whereas in shRNA strategy the presence of the Tal1 gene and/or the persistence of residual TAL1 protein may prevent the activation of such mechanism. Accordingly, normal erythropoiesis has recently been shown to be restored 6 months after the loss of TAL1 in condtal1–/– mice and the authors suggested that alternate factors could replace the essential functions of TAL1.37 Following this idea, we measured the TAL1 close-related bHLH factor LYL1 expression and found it neither decreased nor increased in shRNATAL1 CD34+ cells. This result thus excludes a possible interference of shRNATAL1 on LYL1 expression level that could have explained the decreased reconstitution ability of shRNATAL1-expressing cells, as recently described in lyl–/– mice.38 This result also indicates that normal LYL1 expression is not sufficient to compensate the effects of TAL1's decreased expression. Finally, the residual levels of TAL1 protein expression in the shRNA strategy may indicate that TAL1 can act in a dose-dependent manner as recently described in young zebra fish,31 or as shown for other transcription factors such as GATA-2.39
In conclusion, TAL1 appears as a key regulator of hematopoiesis, not only in erythropoiesis as previously described but also in the normal hematopoietic development from stem cells to committed myeloid progenitors, except in restricted lymphoid cells.
Prepublished online as Blood First Edition Paper, July 18, 2006; DOI 10.1182/blood-2006-05-022988.
Supported by grants from the Ligue Nationale Contre le Cancer (LNCC; RAB05013KKA), INSERM (ASE04144/AMA03019), and Agence Nationale pour la Recherche (ANR) (NT05-2-44232). P.B.G. is a fellow of Fondation de France (FDF), F.A. is supported by a fellowship from LNCC, V.D. was a fellow of the Fondation pour la Recherche Médicale.
P.B.G. and F.P. designed and performed research, analyzed data, and wrote the paper; F.A., V.D. and M.-C.R. performed research; N.G. provided important reagents; and P.-H.R. participated in the writing of the paper.
F.A. and V.D. are co-second authors.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We acknowledge C. Francastel and V. Baud for critical reading of the manuscript, A. Dubart-Kupperschmitt and B. Izac for their expertise in shRNA lentiviral vectors, and S. Fichelson and his “baeckoeffe” band for good scientific lab meetings. We are grateful to the staff of INSERM U602 animal colony, Villejuif, for excellent support in mouse studies and to the midwives of Clinique des Noriets, Vitry-sur-Seine, for sampling cord bloods. We also thank M. Girault from Institut Cochin SCEA. Cell sorting was performed in the common facilities of Institut Cochin.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal