Abstract
We have investigated the in vivo safety, efficacy, and persistence of autologous Epstein Barr virus (EBV)–specific cytotoxic T lymphocytes (CTLs) for the treatment of solid organ transplant (SOT) recipients at high risk for EBV-associated posttransplantation lymphoproliferative disease (PTLD). EBV-CTLs generated from 35 patients expanded with normal kinetics contained both CD8 and CD4 lymphocytes and produced significant specific killing of autologous EBV-transformed B lymphoblastoid cell lines (LCLs). Twelve SOT recipients at high risk for PTLD, or with active disease, received autologous CTL infusions without toxicity. Real-time polymerase chain reaction (PCR) monitoring of EBV-DNA showed a transient increase in plasma EBV-DNA suggestive of lysis of EBV-infected cells, although there was no consistent decrease in virus load in peripheral-blood mononuclear cells. Interferon-γ enzyme-linked immunospot (ELISPOT) assay and tetramer analysis showed an increase in the frequency of EBV-responsive T cells, which returned to preinfusion levels after 2 to 6 months. None of the treated patients developed PTLD. One patient with liver PTLD showed a complete response, and one with ocular disease has had a partial response stable for over one year. These data are consistent with an expansion and persistence of adoptively transferred EBV-CTLs that is limited in the presence of continued immunosuppression but that nonetheless produces clinically useful antiviral activity.
Introduction
One of the major drawbacks of the immunosuppressive regimens employed to prevent allograft rejection is the increased incidence of viral infections and tumors, including Epstein-Barr virus–associated posttransplantation lymphoproliferative disease (EBV-PTLD).1,2
Therapeutic options for EBV-PTLD aim to reduce the tumor burden with antiviral agents,3 cytotoxic drugs,4,5 or monoclonal antibodies6 or to increase immune function by reducing medical immunosuppression.7 Although remissions can be obtained with all these approaches, the recurrence of disease after drug or antibody administration and the risk of graft rejection with reduction of immunosuppression serve to limit the value of each.7,8 Once patients have failed therapy, the clinical outcome is poor and innovative strategies are required.
Infusions of ex vivo–expanded donor-derived EBV-specific cytotoxic T lymphocytes (CTLs) have proved successful in preventing and treating EBV-PTLD in allogeneic stem cell transplant recipients.9-11 Similarly, HLA-matched unrelated donor EBV-CTLs may be of value in patients with EBV-PTLD after solid organ transplantation, although the value of this strategy may be limited by the rapid clearance of allogeneic CTLs from the peripheral blood.12,13 Autologous, transplant recipient–derived EBV-CTLs could represent an alternative means of restoring viral-specific T-cell–mediated immunity after solid organ transplantation, without the need to withdraw immunosuppression and thus globally activate potentially harmful T cells, thereby putting the transplant in jeopardy.14-17 In this study we first evaluated the feasibility of routine manufacture of recipient-derived EBV-CTLs from 35 solid organ allograft recipients who were receiving immunosuppressive drugs. After T-cell generation, we investigated the immunologic, virologic, and clinical consequences of infusing these cells in solid organ transplant (SOT) patients who were at high risk for the development of PTLD or who had confirmed disease. The effects of single- and multiple-dose regimens of CTLs were analyzed. Our results show that EBV-CTLs can routinely be made from SOT recipients even while they are receiving immunosuppression and that infusion of these cells is safe and has effects consistent with immunologic and clinical benefit.
Patients, materials, and methods
Approved protocols for patient enrollment
Patients were eligible for CTL generation if they were considered at high risk for the development of PTLD due to elevated EBV in the peripheral blood (> 1000 copies/μg DNA), had early EBV seroconversion after transplantation, or had confirmed PTLD. The protocol for CTL injection was approved by the Institutional Review Board (IRB) and Ethical Review Committees at Baylor College of Medicine (BCM) Houston, Texas, and at the Charité–Universitätsmedizin Berlin, Germany, and by the Food and Drug Administration (FDA). Patients were eligible to receive CTLs if they had (1) given consent, (2) an EBV load of more than 1000 copies/μg of peripheral-blood DNA in 2 consecutive determinations, (3) biopsy-proven PTLD, (4) life expectancy longer than 6 months, and (5) no evidence of graft rejection. Patients at BCM (n = 8) were treated on a phase 1 dose-escalation protocol, receiving a single injection of 2 × 107 CTLs/m2 (dose level 1), 5 × 107 CTLs/m2 (dose level 2), or 1 × 108 CTLs/m2 (dose level 3). Patients treated in Germany (n = 4) received 3 to 4 consecutive injections (2 weeks apart) of CTLs at a fixed dose of 5 × 107/m2 CTLs. During treatment with CTLs, immunosuppressive treatment was not reduced.
LCL and EBV-CTL line generation
EBV-transformed B lymphoblastoid cell lines (LCLs) and EBV-CTLs were generated from peripheral-blood mononuclear cells, as previously reported.9,18 Briefly, 5 × 106 peripheral-blood mononuclear cells (PBMCs) were incubated with concentrated supernatant of B95-8 cultures in the presence of 1 μg/mL cyclosporin A (Sandoz, Vienna, Austria) to establish an LCL. For CTLs, PBMCs (2 × 106 per well of a 24-well plate) were stimulated with LCLs irradiated at 40 cGy at an effector-stimulator (E/S) ratio of 40:1. After 9 to 12 days, viable cells were restimulated with irradiated LCLs (at a 4:1 E/S ratio). Subsequently, CTLs were expanded by weekly stimulation with LCLs (at a 4:1 E/S ratio) in the presence of recombinant human interleukin-2 (rhIL-2; 40-100 U/mL; Proleukin; Chiron, Emeryville, CA) supplied twice weekly.
After expansion, CTLs were tested for sterility, identity by HLA typing, immunophenotype, and EBV specificity and cryopreserved. Specificity was tested in a 4-hour Cr51 release assay.9,18 Autoreactivity was excluded by the absence of lysis of autologous phytohemagglutinin (PHA)–stimulated lymphoblasts. To test for organ-specific reactivity, PHA-stimulated lymphocytes were generated from PBMCs from the organ donor, whenever available. The EBV specificity of the CTL lines was also evaluated using interferon-gamma (IFN-γ enzyme-linked immunospot (ELISPOT) assay and specific peptide-HLA tetramers.19,20
Peptides and tetramer staining
The following peptides were used for analysis of EBV-specific T-cell populations according to the patients' HLA specificity: EBNA1, HLA-B35: HPVGEADYFEY; EBNA2, HLA-A2: DTPLIPLTIF; EBNA3A, HLA-A2: SVRDRLARL; HLA-A3: RLRAEAQVK; HLA-B7: RPPIFIRLL, VPAPAGPIV; HLA-B8: QAKWRLQTL, FLRGRAYGL; HLA-B35: YPLHEQHGM; EBNA3B, HLA-A11: AVFDRKSDAK, IVTDFSVIK, LPGPQVTAVLLHHEES, DEPASTEPVHDQLL, NPTQAPVIQLVHAVY; HLA-A24: TYSAGIVQI; HLA-B35: AVLLHEESM; HLA-B44: VEIT-PYKPTW; EBNA3C, HLA-A2: LLDFVRFMGV; HLA-B27: FRKAQIQGL; HLA-B44: EENLLDFVRF; LMP2, HLA-A2: CLGGLLTMV; HLA-A11: SSCSSCPLSKI; HLA-A24: TYGPVFMCL; HLA-B27: RRRWRRLTV; HLA-35: MGSLEVMPM; BZLF1, HLA-B8: RAKFKQLL; HLA-B35: EPLPQGQLTAY; BRLF1, HLA-A2: YVLDHLIVV; HLA-A3: RVRAYTYSK, KHSRVRAYTYSK; HLA-A11: ATIGTAMYK; HLA-A24: DYCNVLNKEF; BMLF1, HLA-A2: GLCTLVAML; and BMRF1, HLA-A2: TLDYKPLSV (listed in Khanna and Burrows21 and Houssaint et al22 ). Peptides were synthesized by either Martin Campbell (Synthetic Antigen Laboratory, The University of Texas M. D. Anderson Cancer Center, Houston, TX) or Genemed Synthesis (South San Francisco, CA). In this paper, the peptides are referred to by the first 3 amino acids as underlined. Tetramers were prepared by the Baylor College of Medicine tetramer core facility. CTLs or PBMCs (5 × 105 to 10 × 105) were incubated at room temperature for 30 minutes in PBS/1% FCS containing the PE-labeled tetrameric complex. Samples were costained with anti-CD8 FITC and anti-CD3 PerCP. Appropriate isotype controls were included. Stained cells were fixed in PBS containing 0.5% paraformaldehyde. For each sample, a minimum of 100 000 cells was analyzed using a FACSCalibur with CellQuest software (BD Biosciences).
Treatment and monitoring
All patients enrolled in the study were monitored clinically for the occurrence of adverse events and graft rejection. Computerized tomography was performed in patients with clinical symptoms suggestive of PTLD. Biopsies were performed of suspected sites and analyzed using conventional histologic examination and immunophenotyping.
To evaluate the antiviral activity of CTL infusion, we monitored the EBV-DNA load in PBMCs and in plasma samples collected sequentially before and after CTL infusion. DNA was isolated from 3 × 106 to 5 × 106 PBMCs or 200 μL plasma using an anion exchange column (Qiagen, Valencia, CA). The isolated DNA was then used to estimate the EBV-DNA copy number using our previously validated real-time polymerase chain reaction (PCR).18
To measure the effect of CTL infusion on the EBV-specific CTL precursor (CTLp) frequency, we used a specific IFN-γ ELISPOT assay to enumerate the cells producing IFN-γ in response to autologous LCL stimulation.19 PBMCs were collected before CTL infusion and at 2, 4, and 6 weeks after administration and then at monthly intervals. The PBMCs were stored frozen and all samples were analyzed in a single assay. Briefly, MAHA S45 plates (Millipore, Bedford, MA) were coated with anti–IFN-γ antibody 1 DIK (Mabtech, Mariemont, OH) overnight and blocked with complete medium for 1 hour at 37°C. Thawed PBMCs were added at doubling dilution from 1 × 105/well (3 replicates for each dilution) in the presence of autologous LCLs for 24 hours at 37°C. After washing, the plates were incubated for 2 hours at 37°C with biotin anti–IFN-γ antibody 7-B6-1 (Mabtech). Controls consisting of PBMCs, LCLs, and medium alone were also plated and incubated with biotin anti–IFN-γ antibody 7-B6-1. Avidin-peroxidase-complex (Vector Laboratories, Burlingame, CA) was added for 1 hour at room temperature and spots were developed with 3-amino-9-ethylcarbazole (AEC; Sigma, St Louis, MO) substrate mix. Spots were counted (Zellnet Consulting, New York, NY) and expressed as the number of spot-forming cells (SFCs)/105 cells when dilution was linear. Negligible numbers of spots (< 5) were produced by medium, LCLs, or PBMCs alone. In 2 patients whose CTLs predominantly consisted of CD4+ cells, the ELISPOT assay was performed with PBMCs enriched with CD4 or CD8 microbeads (Miltenyi, Auburn, CA).
Statistical analysis
Data are shown as mean ± SD. Significance was assessed by paired t testing, and results were considered significant at P values less than .05.
Results
Patient details
Between January 1999 and September 2004, 35 SOT recipients (12 female and 23 male) considered at “high risk” for development of PTLD were enrolled for the generation of autologous EBV-CTLs. The reasons for the enrolment in the CTL generation protocol were (1) persisting high EBV-DNA viral load with no evidence of PTLD (23 patients, 66%), (2) high EBV-DNA load with previous or current clinical diagnosis of PTLD (8 patients, 23%), or (3) early posttransplantation EBV seroconversion (4 patients, 11%). Thirty-one (88%) patients were children (< 18 years). Twenty-five patients (72%) received liver transplants, 6 received hearts (28%), and 4 (11%) received kidneys. Twenty-five patients (69%) were receiving tacrolimus (FK506) and 10 patients (31%) were receiving cyclosporin A (CsA) at the time of enrolment. For 26 patients (74%), CTLs were initiated within 2 years of transplantation.
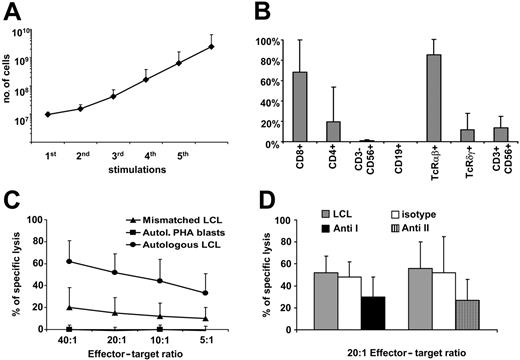
Characteristics of patient CTL lines. The growth kinetics, immunophenotype, and cytolytic properties of the CTL lines generated from our cohort of SOT recipients are shown. EBV-CTL lines were expanded from PBMCs of 35 SOT patients by weekly stimulations with irradiated LCLs and biweekly feeding with IL-2. (A) The mean ± SD of the growth rate of all 35 CTL lines generated. (B) The majority of the CTL lines were CD3+CD8+ and TCRαβ-positive T cells. Data are shown as the mean % of positive cells ± SD. (C) The cytotoxic activity of these expanded lines. Lysis of autologous LCLs (•) is significantly higher compared with lysis of HLA-mismatched LCLs (▴). Autoreactivity was excluded by absence of lysis of autologous PHA blasts (▪). Shown is the mean Cr51 release of the CTL lines ± SD. (D) Killing of autologous LCLs (▦) is inhibited after preincubation with anti–class I (▪) and anti–class II (▥) blocking antibodies of CTLs prevalently composed of CD8+ T cells (left bars) and of CTLs prevalently composed of CD4+ T cells (right bars). □ shows lysis in the presence of isotype control mAbs. Bars indicate mean Cr51 release of CTLs ± SD.
Characteristics of patient CTL lines. The growth kinetics, immunophenotype, and cytolytic properties of the CTL lines generated from our cohort of SOT recipients are shown. EBV-CTL lines were expanded from PBMCs of 35 SOT patients by weekly stimulations with irradiated LCLs and biweekly feeding with IL-2. (A) The mean ± SD of the growth rate of all 35 CTL lines generated. (B) The majority of the CTL lines were CD3+CD8+ and TCRαβ-positive T cells. Data are shown as the mean % of positive cells ± SD. (C) The cytotoxic activity of these expanded lines. Lysis of autologous LCLs (•) is significantly higher compared with lysis of HLA-mismatched LCLs (▴). Autoreactivity was excluded by absence of lysis of autologous PHA blasts (▪). Shown is the mean Cr51 release of the CTL lines ± SD. (D) Killing of autologous LCLs (▦) is inhibited after preincubation with anti–class I (▪) and anti–class II (▥) blocking antibodies of CTLs prevalently composed of CD8+ T cells (left bars) and of CTLs prevalently composed of CD4+ T cells (right bars). □ shows lysis in the presence of isotype control mAbs. Bars indicate mean Cr51 release of CTLs ± SD.
EBV-CTL generation and characterization
Although cells were obtained for CTL generation at a time when the patients were receiving immunosuppressive drugs, the kinetics of EBV-CTL growth was comparable to that of CTLs generated from healthy donors (Figure 1A). The mean time required to achieve numbers of CTLs sufficient for at least 2 doses of 5 × 107/m2 was 42.6 days (median, 41 days; range, 30-70 days; mean for healthy donors 38 days, median 39 days).
The majority of the cells in the EBV-CTL lines generated from these patients were CD3+CD8+ (68% ± 32%). In 3 patients, however, greater than 90% were CD3+CD4+. TCRγδ-positive cells were generally less than 8% of the T-cell population but formed greater than 40% of CD3+ cells in 3 patients. No residual B cells (< 1%) could be detected in any of the expanded lines at the time the cells were frozen for infusion. Natural killer cells (CD3–CD56+/CD16+) were 3% ± 4% of the total, whereas lymphokine-activated killer (LAK) cells (CD3+CD56+) were 17% ± 14% (Figure 1B).
The EBV specificity of these CTL lines was confirmed by their preferential lysis of autologous LCLs (57% ± 16%, at a 20:1 effector-target [E/T] ratio) compared with allogeneic LCLs (14% ± 13%) when tested in a standard chromium release assay (Figure 1C). In addition, killing by CD8+ CTL lines was significantly inhibited by preincubation of the target cells with HLA class I–blocking antibody (30% ± 18%; P < .01; Figure 1D). Killing by the 3 CD4+ CTL lines was inhibited by preincubation with anti–class II–blocking antibody (27% ± 19%; P < .03; Figure 1D). Hence, the cytotoxicity of both CD8+ and CD4+ lines were major histocompatibility complex (MHC) restricted. The lysis of the HSB-2 cell line, sensitive to LAK cells, was 30% ± 24%. Killing of both autologous and donor-derived PHA blasts (when available) was less than 10% at a 20:1 E/T ratio (mean 1% ± 1%). Of all the lines generated, only 2 lines (both from EBV-seronegative donor/recipient pairs) had no significant killing of the autologous LCL target (< 10% at 20:1 E/T ratio) and would therefore not have met release criteria for infusion. Thus, in 94% (33/35) of patients on immunosuppressive drugs, we could generate EBV-CTL lines suitable for infusion, underlining the feasibility and high reproducibility of this approach in SOT recipients.
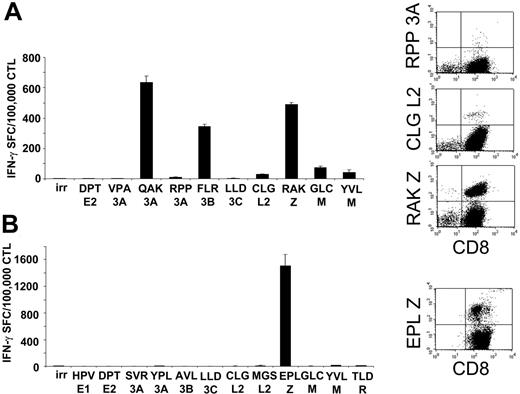
To ensure that these CTLs had the broad EBV reactivity likely necessary for effective activity,23 we analyzed the EBV epitope specificities of 17 lines using specific peptides and IFN-γ ELISPOT assays. We considered the response to EBV epitopes to be significant if more than 100 IFN-γ SFCs were detected per 105 CTLs. Table 1 shows that 13 of 17 of the CTLs recognized lytic EBV antigens, since they reacted with peptides derived from early and immediate-early lytic proteins. Responses to latent EBV antigens were also detected, with 25% and 37% of the lines recognizing EBNA3A and EBNA3B, respectively. Five (29%) of the 17 lines responded to LMP-2 epitopes. For 4 donors, the CTL lines could also be analyzed with tetramers, which confirmed the expected hierarchy of EBV epitope reactivity (lytic > latent; Figure 2). Analysis of healthy EBV-seropositive donors confirmed a similar lytic greater than latent epitope hierarchy (data not shown).
Analysis of EBV specificity within the patient CTL lines
EBV protein . | HLA restriction and epitope sequence . | No. of CTL lines, responders/tested (%) . |
|---|---|---|
| EBNA1 | B35: HPV | 1/3 (33) |
| EBNA2 | A2: DTP | 0/9 (0) |
| EBNA3A | A2: SVR; A3: RLR; B7: RPP, VPA; B8: QAK, FLR; B35: YPL | 4/16 (25) |
| EBNA3B | A11: AVF, IVT, LPG, DEP, NPT; A24: TYS; B35: AVL; B44: VEI | 3/8 (37.5) |
| EBNA3C | A2: LLD; B27: FRK; B44: EEN | 0/13 (0) |
| LMP2 | A2: CLG; A11: SSC; A24: TYG; B27: RRR; B35: MSG | 5/17 (29) |
| BMLF1, BZLF1, BRLF1 | A2: GLC, YVL, TLD; A3: RVR, KHS; A11: ATI; A24: DYC; B8: RAK; B35: EPL | 13/17 (76) |
EBV protein . | HLA restriction and epitope sequence . | No. of CTL lines, responders/tested (%) . |
|---|---|---|
| EBNA1 | B35: HPV | 1/3 (33) |
| EBNA2 | A2: DTP | 0/9 (0) |
| EBNA3A | A2: SVR; A3: RLR; B7: RPP, VPA; B8: QAK, FLR; B35: YPL | 4/16 (25) |
| EBNA3B | A11: AVF, IVT, LPG, DEP, NPT; A24: TYS; B35: AVL; B44: VEI | 3/8 (37.5) |
| EBNA3C | A2: LLD; B27: FRK; B44: EEN | 0/13 (0) |
| LMP2 | A2: CLG; A11: SSC; A24: TYG; B27: RRR; B35: MSG | 5/17 (29) |
| BMLF1, BZLF1, BRLF1 | A2: GLC, YVL, TLD; A3: RVR, KHS; A11: ATI; A24: DYC; B8: RAK; B35: EPL | 13/17 (76) |
Infusion of EBV-CTLs
Between August 2001 and August 2004, 12 of the 33 lines made from “high-risk” patients were infused. Table 2 summarizes the characteristics of the patients who met the criteria for CTL infusion. Of the remaining 21 individuals, CTL infusion was deferred because (1) seroconversion early after transplantation was not followed by a rising EBV load (n = 2); (2) PTLD resolved and did not relapse after chemotherapy and rituximab (n = 5); (3) high viral load resolved after reduction of immunosuppression (n = 9); (4) graft rejection (n = 3); or (5) individual developed PTLD but elected not to consent to experimental treatment (n = 2, with 1 death).
Characteristics of the patients receiving T-cell infusions
Patient . | Sex/age at Tx, y . | Tx . | Immuno-suppression . | Time from Tx to high viral load, mo . | EBV-DNA load . | Time from Tx to infusion, mo . | Previous history of PTLD . |
|---|---|---|---|---|---|---|---|
| 1 | F/3.1 | Liver | FK506 | 11 | 4 156 | 13 | No |
| 2 | F/1.1 | Liver | FK506 | 4 | 136 000 | 9.2 | No |
| 3 | M/1 | Liver | FK506 | 18 | 4 506 | 22 | Yes, adenoid: polymorphic, EBV+; removed 2 mo prior to CTL infusion |
| 4 | M/0.7 | Liver | FK506 | 4 | 16 204 | 18 | Yes, LN: PTLD of intermediated grade EBV+; removed 1 y prior to CTL infusion |
| 5 | F/0.6 | Liver | FK506 | 4 | 10 172 | 8 | No |
| 6 | M/1.6 | Liver | FK506 | 9 | 6 704 | 17 | Yes, diffuse lymphadenopathy: rituximab 5 mo prior to CTL infusion |
| 7 | F/3.3 | Liver | FK506 | 4 | 4 938 | 28 | Yes, gut: polymorphic EBV+, CD20+; rituximab 1 y prior to CTL infusion |
| 8 | F/2.1 | Liver | FK506 | 9 | 14 002 | 20 | No |
| 9 | M/3.5 | Heart | CsA | 60 | 2 500 | 68 | Yes, ocular PTLD; irradiation but loss of vision 1 y prior to CTL infusion |
| 10 | M/40 | Heart | CsA | 4 | 1 100 | 11 | No |
| 11 | F/1.1 | Heart | CsA | 142 | 1 500 | 155 | No |
| 12 | M/3.3 | Heart | CsA | 35 | 20 900 | 43 | Yes, ocular PTLD |
Patient . | Sex/age at Tx, y . | Tx . | Immuno-suppression . | Time from Tx to high viral load, mo . | EBV-DNA load . | Time from Tx to infusion, mo . | Previous history of PTLD . |
|---|---|---|---|---|---|---|---|
| 1 | F/3.1 | Liver | FK506 | 11 | 4 156 | 13 | No |
| 2 | F/1.1 | Liver | FK506 | 4 | 136 000 | 9.2 | No |
| 3 | M/1 | Liver | FK506 | 18 | 4 506 | 22 | Yes, adenoid: polymorphic, EBV+; removed 2 mo prior to CTL infusion |
| 4 | M/0.7 | Liver | FK506 | 4 | 16 204 | 18 | Yes, LN: PTLD of intermediated grade EBV+; removed 1 y prior to CTL infusion |
| 5 | F/0.6 | Liver | FK506 | 4 | 10 172 | 8 | No |
| 6 | M/1.6 | Liver | FK506 | 9 | 6 704 | 17 | Yes, diffuse lymphadenopathy: rituximab 5 mo prior to CTL infusion |
| 7 | F/3.3 | Liver | FK506 | 4 | 4 938 | 28 | Yes, gut: polymorphic EBV+, CD20+; rituximab 1 y prior to CTL infusion |
| 8 | F/2.1 | Liver | FK506 | 9 | 14 002 | 20 | No |
| 9 | M/3.5 | Heart | CsA | 60 | 2 500 | 68 | Yes, ocular PTLD; irradiation but loss of vision 1 y prior to CTL infusion |
| 10 | M/40 | Heart | CsA | 4 | 1 100 | 11 | No |
| 11 | F/1.1 | Heart | CsA | 142 | 1 500 | 155 | No |
| 12 | M/3.3 | Heart | CsA | 35 | 20 900 | 43 | Yes, ocular PTLD |
Tx indicates transplant; F, female; FK506, tacrolimus; M, male; LN, lymph node; and CsA, cyclosporin A.
Of the 12 treated patients, 8 received a single dose of cells on the dose-escalation study at BCM. The remaining 4 received 3 to 4 consecutive infusions of 5 × 107 CTLs/m2 in a study at Institute Charité Berlin, with the intent of assessing the effects of a multi-dose regimen. The median time from transplantation to CTL infusion was 1.7 years (range, 0.6-12 years). Immunosuppression was not reduced prior to infusion to minimize risks of graft rejection, and trough levels of FK506 and CsA were maintained at circa 10 ng/mL and 100 ng/mL, respectively. None of the patients were receiving steroids at the time of CTL infusion. One patient had clinical evidence of (ocular) PTLD at the time of infusion, but 5 others were in apparent clinical remission of localized PTLD (treated by surgical excision/radiotherapy or rituximab) while continuing to have a persistently high EBV-DNA viral load.
Spectrum of EBV antigen specificity of the CTL lines. The majority of the CTLs generated from SOT patients are directed against lytic EBV antigens (Table 2). Two representative CTL lines analyzed for the presence of EBV-latent and -lytic specificities with IFN-γ ELISPOT assay and tetramers are shown. For IFN-γ ELISPOT assay (left panels), CTLs (1 × 105/well) were stimulated with a panel of peptides representing described epitopes that were informative based on the patients' HLA type. Results are shown as the mean of triplicate wells ± SD. Right panels show the same CTL lines tested using EBV-specific tetramers available for these patients based on their HLA type. Peptides are described in “Patients, materials, and methods,” under “Peptides and tetramer staining.” irr indicates irrelevant peptide.
Spectrum of EBV antigen specificity of the CTL lines. The majority of the CTLs generated from SOT patients are directed against lytic EBV antigens (Table 2). Two representative CTL lines analyzed for the presence of EBV-latent and -lytic specificities with IFN-γ ELISPOT assay and tetramers are shown. For IFN-γ ELISPOT assay (left panels), CTLs (1 × 105/well) were stimulated with a panel of peptides representing described epitopes that were informative based on the patients' HLA type. Results are shown as the mean of triplicate wells ± SD. Right panels show the same CTL lines tested using EBV-specific tetramers available for these patients based on their HLA type. Peptides are described in “Patients, materials, and methods,” under “Peptides and tetramer staining.” irr indicates irrelevant peptide.
None of the treated patients had any immediate organ toxicity or signs of graft rejection after CTL infusion, suggesting the safety of our approach. There were no other short- or long-term adverse events (Table 3).
Outcome of treatment
. | Before CTL evaluation . | . | . | . | Fold reduction in EBV load . | . | Outcome at 1 y . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | EBV DNA load . | PTLD . | CTL dose . | Toxicity . | 2 mo after . | 6 mo after . | Clinical . | EBV DNA load . | |||
| 1 | 4 156 | No | 2 × 107/m2 | No | 2.3 | 0.7 | Well | 1 500 | |||
| 2 | 136 000 | No | 2 × 107/m2 | No | 0.6 | 3 | Well | 180 000 | |||
| 3 | 4 506 | No | 2 × 107/m2 | Transient rise in AST* | 2.5 | 3.7 | Well | 400 | |||
| 4 | 16 204 | No | 5 × 107/m2 | No | 0.1 | 1 | Well | 15 000 | |||
| 5 | 10 172 | No | 5 × 107/m2 | No | 0.4 | 1.3 | Well | 3 900 | |||
| 6 | 6 704 | No | 5 × 107/m2 | No | 0.6 | 10 | Well | 1 100 | |||
| 7 | 4 938 | No | 1 × 108/m2 | No | 0.9 | 0.6 | Well | 3 900 | |||
| 8 | 14 002 | No | 1 × 108/m2 | No | 0.6 | 0.9 | Well | 7 200 | |||
| 9 | 2 500 | No | 5 × 107/m2 × 3 | No | 1.7 | 10 | Well | 7 000 | |||
| 10 | 1 100 | No | 5 × 107/m2 × 3 | No | 2.7 | 10 | Well | < 400 | |||
| 11 | 1 500 | No | 5 × 107/m2 × 4 | No | 0.4 | 1.2 | Well | 700 | |||
| 12 | 20 900 | Eye | 5 × 107/m2 × 4 | No | 0.8 | 3 | Well† | 25 000 | |||
. | Before CTL evaluation . | . | . | . | Fold reduction in EBV load . | . | Outcome at 1 y . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | EBV DNA load . | PTLD . | CTL dose . | Toxicity . | 2 mo after . | 6 mo after . | Clinical . | EBV DNA load . | |||
| 1 | 4 156 | No | 2 × 107/m2 | No | 2.3 | 0.7 | Well | 1 500 | |||
| 2 | 136 000 | No | 2 × 107/m2 | No | 0.6 | 3 | Well | 180 000 | |||
| 3 | 4 506 | No | 2 × 107/m2 | Transient rise in AST* | 2.5 | 3.7 | Well | 400 | |||
| 4 | 16 204 | No | 5 × 107/m2 | No | 0.1 | 1 | Well | 15 000 | |||
| 5 | 10 172 | No | 5 × 107/m2 | No | 0.4 | 1.3 | Well | 3 900 | |||
| 6 | 6 704 | No | 5 × 107/m2 | No | 0.6 | 10 | Well | 1 100 | |||
| 7 | 4 938 | No | 1 × 108/m2 | No | 0.9 | 0.6 | Well | 3 900 | |||
| 8 | 14 002 | No | 1 × 108/m2 | No | 0.6 | 0.9 | Well | 7 200 | |||
| 9 | 2 500 | No | 5 × 107/m2 × 3 | No | 1.7 | 10 | Well | 7 000 | |||
| 10 | 1 100 | No | 5 × 107/m2 × 3 | No | 2.7 | 10 | Well | < 400 | |||
| 11 | 1 500 | No | 5 × 107/m2 × 4 | No | 0.4 | 1.2 | Well | 700 | |||
| 12 | 20 900 | Eye | 5 × 107/m2 × 4 | No | 0.8 | 3 | Well† | 25 000 | |||
Two months after CTL infusion.
PTLD decrease in size.
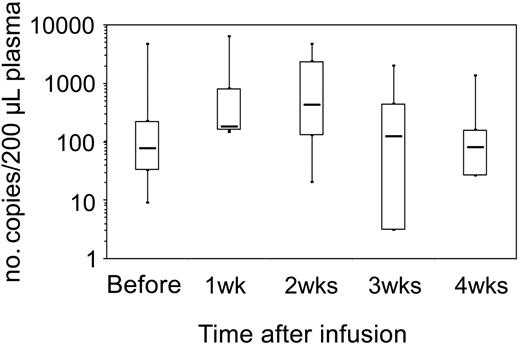
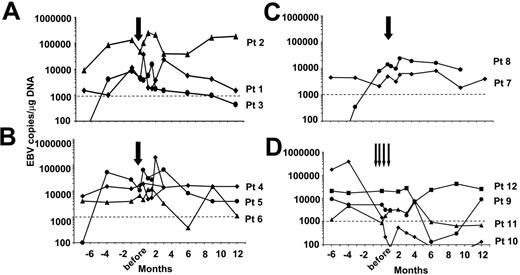
Effects on EBV-DNA. The antiviral effects of the adoptive transferred lines were studied by monitoring EBV-DNA in the PBMC and (for the 8 patients treated in the single-dose study) in the plasma. At study entry, patients had EBV load ranging from 1100 to 136 000 copies/μg of DNA in their peripheral blood prior to CTL infusion. Analysis of plasma showed a transient increase of free EBV-DNA within 2 weeks of infusion (Figure 3), consistent with lysis of EBV-infected B cells. However, there was no consistent reduction in the total peripheral-blood EBV load with single-dose infusion, even when higher numbers of CTLs were infused (Figure 4A-C). Similarly, the multiple-infusions regimen induced a substantial fall in the EBV-DNA in only 1 of 4 patients (no. 10), whose EBV load fell to fewer than 400 copies after CTL administration (Figure 4D).
The viral DNA detected after CTL transfer was not due to contamination of the CTL product with the laboratory strain of EBV, since PCR amplification assays using primers specific for the B95-8 strain, which has a characteristic 12-kbp deletion,24 were consistently negative.
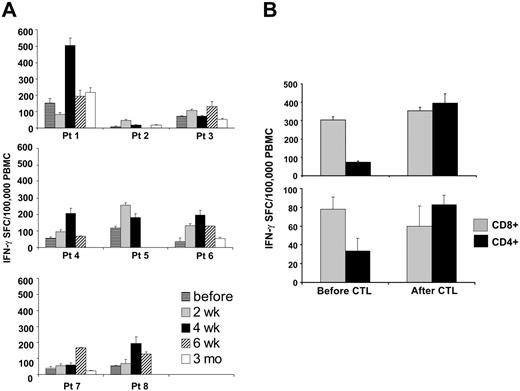
Effects on EBV–specific cytotoxic T-cell numbers. To assess the expansion and persistence of the infused CTLs, we measured the frequency of EBV-specific IFN-γ–secreting cells produced in response to autologous LCLs, using a specific ELISPOT assay. Prior to infusion, all patients treated with a single dose of CTLs had low frequency of EBV-specific IFN-γ–producing cells. After adoptive transfer, there was a 1.5- to 4.8-fold increase in the frequency of these cells (Figure 5A). Patients no. 1 and no. 3 received CTLs containing greater than 90% CD3+CD4+ cells and, in these 2 patients, the increase occurred only in the CD4+ T-cell population, whereas the frequency of IFN-γ–positive CD8+ T cells remained unchanged (Figure 5B). In all patients, the frequency of EBV-CTLs returned to preinfusion levels within 2 months of single-dose CTL infusion (Figure 5A). There was no discernible difference in T-cell expansion or persistence between dose levels.
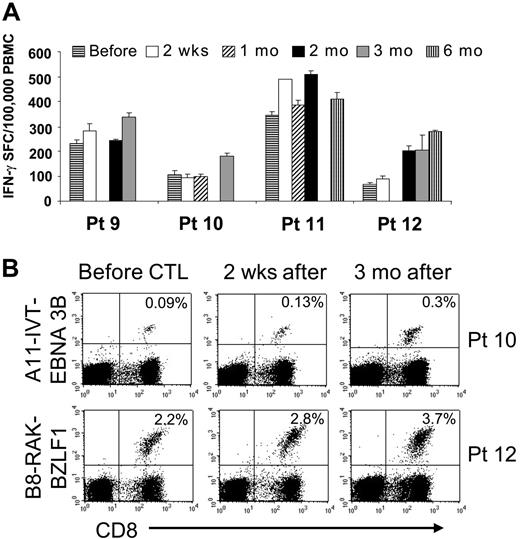
Patients treated with multiple doses of EBV-CTLs had a similar increase in the frequency of EBV-responsive T cells (Figure 6A). Although the increase was additive after each infusion, persistence was prolonged only in patient no. 12, in whom the frequency of EBV-specific T cells remained elevated for 6 months. In 2 of the 4 multidose patients, tetramers were also available to measure antigen binding by the T cells (EBNA3B tetramer for patient no. 10 and BZLF1 for patient no. 12). The frequency of CTLs reacting to the immunodominant EBV antigens increased after infusion and persisted 1.5- to 3-fold higher 3 months after termination of CTL infusion (Figure 6B).
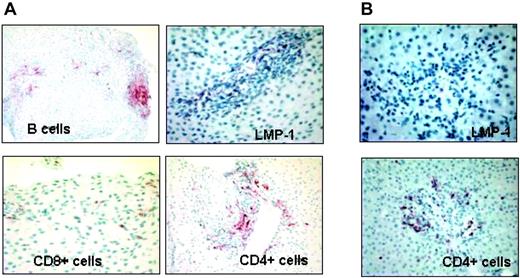
Clinical effects. Ten of 12 patients had no evidence of overt PTLD following CTL therapy, despite being categorized as “high risk.” The 2 remaining patients both had evidence of pre-existing PTLD (one diagnosed before infusion, the other immediately after) and both appeared to respond to CTLs. Patient no. 3 (Table 3) developed a transaminitis 8 weeks after single-dose CTL infusion (aspartate aminotransferase/alanine amintotransferase [AST/ALT] from 32/33 pre-CTLs to 197/367; 20-60/5-45 U/L normal range, respectively). To exclude graft rejection, we performed a liver biopsy. Immunohistochemistry showed infiltration of EBV (LMP1)–positive B cells with massive coinfiltration of CD4+ T cells surrounding the EBV-positive areas. This pattern was interpreted as pre-existing PTLD with hepatic involvement, followed by CTL-mediated inflammation (Figure 7A). Consistent with this interpretation, liver enzymes normalized within 4 weeks (AST/ALT 54/45 U/L) and repeat biopsy showed complete disappearance of the LMP1-positive B-cell infiltrate and a reduction in infiltrating CD4+ T cells (Figure 7B). Patient no. 12 (multiple-dose infusion) had primary PTLD localized to his left eye on study entry, which presented as a nodular structure of 3 mm × 4 mm with surrounding uveitis. This lesion decreased by 50% after infusion and has remained stable for over a year. No new lesions have appeared. Consent to repeat biopsy was not obtained.
Discussion
In this study we were able to successfully generate EBV-CTLs from 33 of 35 solid organ transplant recipients while they were receiving immunosuppressive drugs. We infused these autologous ex vivo–expanded EBV-CTLs in 12 of the recipients who had persisting high EBV-DNAload and/or localized PTLD. T-cell transfer was safe, as there was no graft rejection or other toxicities. Infusion also increased the number of EBV-responsive T cells in the circulation and controlled PTLD in 2 patients with this complication.
In vivo lysis of EBV-infected B cells by EBV-CTLs. Lysis of EBV-infected B cells occurred after CTL infusion. For this purpose, free EBV-DNA was detected in the plasma by real-time PCR before and after infusion. The number of EBV-DNA copies in 200 μL plasma is shown on the y-axis. A transient increase of free EBV-DNA in the plasma was observed within 2 weeks after CTL infusion, consistent with lysis of EBV-infected B cells. Each box shows the median (bar), upper, and lower quartiles and range.
In vivo lysis of EBV-infected B cells by EBV-CTLs. Lysis of EBV-infected B cells occurred after CTL infusion. For this purpose, free EBV-DNA was detected in the plasma by real-time PCR before and after infusion. The number of EBV-DNA copies in 200 μL plasma is shown on the y-axis. A transient increase of free EBV-DNA in the plasma was observed within 2 weeks after CTL infusion, consistent with lysis of EBV-infected B cells. Each box shows the median (bar), upper, and lower quartiles and range.
We have previously shown that adoptive transfer of EBV-CTLs to hematopoietic stem cell transplant (HSCT) recipients safely and effectively prevented EBV reactivation and restored long-term immunocompetence to the virus.9-11 Moreover, patients with established EBV-PTLD responded with tumor regression.9 It was uncertain, however, whether the same benefits would be experienced if solid organ transplant recipients were to be treated in the same way. Although these patients also have a high incidence of EBV-PTLD, the presence of continued immunosuppression and the absence of the lymphodepletion that follows HSC transplantation would mitigate against the in vivo expansion and persistence of EBV-CTLs that likely underpin their effectiveness in HSCT recipients. These concerns notwithstanding, a previous report by Khanna et al14 described the infusion of autologous EBV-CTLs in a lung transplant recipient with pulmonary PTLD. After 3 doses of CTLs, the tumor significantly regressed but the patient succumbed to massive bleeding from an eroded pulmonary artery adjacent to the tumor.14 Comoli et al16 infused autologous EBV-CTLs prophylactically to 7 SOT patients with high-level viremia, which resulted in an increase in CTLp frequency and reduction of viral load in 5 of the treated patients. The 2 studies supported the use of CTLs even in SOT recipients receiving immunosuppression but also emphasized the need for early intervention in patients with active disease.
Monitoring of EBV load in SOT recipients after adoptive transfer of EBV-CTLs. The antiviral responses of patients treated with EBV-CTLs during the first year after infusion are shown. The antiviral activity of the CTLs infused was monitored measuring the EBV-DNA viral load in PBMCs by real-time PCR. Panel A shows the EBV-DNA viral load in patients who received one single dose of EBV-CTLs 2 × 107/m2; panel B shows the load in those who received 5 × 107/m2; and panel C shows the load in those treated with 1 × 108/m2 CTLs. Panel D shows EBV-DNA in patients who received multiples doses of 5 × 107/m2 EBV-CTLs. Pt indicates patient. Arrows indicate CTL infusions; dotted lines indicate the cutoff of EBV-DNA load above which patients were eligible for CTL infusion.
Monitoring of EBV load in SOT recipients after adoptive transfer of EBV-CTLs. The antiviral responses of patients treated with EBV-CTLs during the first year after infusion are shown. The antiviral activity of the CTLs infused was monitored measuring the EBV-DNA viral load in PBMCs by real-time PCR. Panel A shows the EBV-DNA viral load in patients who received one single dose of EBV-CTLs 2 × 107/m2; panel B shows the load in those who received 5 × 107/m2; and panel C shows the load in those treated with 1 × 108/m2 CTLs. Panel D shows EBV-DNA in patients who received multiples doses of 5 × 107/m2 EBV-CTLs. Pt indicates patient. Arrows indicate CTL infusions; dotted lines indicate the cutoff of EBV-DNA load above which patients were eligible for CTL infusion.
Immunologic activity of EBV-CTLs. Panel A shows the frequency of EBV-specific T cells in the peripheral blood as measured by the number of IFN-γ–secreting PBMCs upon stimulation with irradiated autologous LCLs in an IFN-γ ELISPOT assay before and after one single dose of CTLs. Bars represent the mean of triplicate wells ± SD. A transient but significant increase of CTL precursors compared with the pretreatment level was observed. Panel B shows in vivo expansion of the infused cells. Patients 1 (top graph) and 3 (bottom graph) received CTLs mainly containing CD3+CD4+ cells. The figure shows the frequency of T cells responding to autologous LCLs assessed by IFN-γ ELISPOT assay on CD4+ (▪) and on CD8+ (▦) selected cells before and 4 and 2 weeks after CTL infusion, respectively. An increase in the number of CD4+ T cells was observed, whereas CD8+ T-cell frequencies remained unchanged.
Immunologic activity of EBV-CTLs. Panel A shows the frequency of EBV-specific T cells in the peripheral blood as measured by the number of IFN-γ–secreting PBMCs upon stimulation with irradiated autologous LCLs in an IFN-γ ELISPOT assay before and after one single dose of CTLs. Bars represent the mean of triplicate wells ± SD. A transient but significant increase of CTL precursors compared with the pretreatment level was observed. Panel B shows in vivo expansion of the infused cells. Patients 1 (top graph) and 3 (bottom graph) received CTLs mainly containing CD3+CD4+ cells. The figure shows the frequency of T cells responding to autologous LCLs assessed by IFN-γ ELISPOT assay on CD4+ (▪) and on CD8+ (▦) selected cells before and 4 and 2 weeks after CTL infusion, respectively. An increase in the number of CD4+ T cells was observed, whereas CD8+ T-cell frequencies remained unchanged.
Based on these experiences, we evaluated the feasibility, safety, and effectiveness of preparing and administering EBV-CTLs in a larger series of SOT recipients (35 patients). We prepared CTLs from high-risk patients including those with a persistently high EBV-DNA viral load, which predicts subsequent PTLD.25 Our initial concern was that our protocol for CTL generation from healthy donors would prove inadequate for T cells obtained from patients on immunosuppressive drugs.9 In fact, expansion times, phenotype, and function of the CTLs produced were all indistinguishable from the pattern observed in healthy donors. Failure occurred in only the 2 patients whose samples were still EBV seronegative at the time of blood collection for CTL generation. Importantly, the CTLs we prepared were reactive with a broad array of EBV antigens, with the anticipated predilection for the immunodominant early lytic cycle and EBNA antigens, which are generally expressed by PTLD.26 This broad T-cell reactivity minimizes the risk that antigen loss mutants will be able to escape destruction by the CTLs.23 We conclude that the effects of the immunosuppressive drugs used in solid organ transplantation have no persisting (ex vivo) effect on virus-specific T cells and in particular that they do not induce clonal anergy or deletion in vivo, should virus-specific T cells encounter their cognate antigen.
Immunologic evaluation of patients treated in the multiple-doses protocol. Four patients received multiple doses of 5 × 107/m2 EBV-CTLs. Panel A shows the frequency of EBV-specific T cells in the peripheral blood as measured by the number of IFN-γ–secreting PBMCs upon stimulation with irradiated autologous LCLs before and after CTL infusion. Bars represent the mean of triplicate wells ± SD. ELISPOT assay shows an increase in the frequency of circulating EBV-specific T cells after CTL infusion, persisting for longer than 2 months after infusion. Panel B shows the immunologic response of 2 patients with informative tetramers in the EBV-CTLs infused. The right panels show the frequency of tetramer-positive T cells on PBMCs collected before infusion. Left panels show that the frequency of the tetramer-positive T cells increased after CTL infusion.
Immunologic evaluation of patients treated in the multiple-doses protocol. Four patients received multiple doses of 5 × 107/m2 EBV-CTLs. Panel A shows the frequency of EBV-specific T cells in the peripheral blood as measured by the number of IFN-γ–secreting PBMCs upon stimulation with irradiated autologous LCLs before and after CTL infusion. Bars represent the mean of triplicate wells ± SD. ELISPOT assay shows an increase in the frequency of circulating EBV-specific T cells after CTL infusion, persisting for longer than 2 months after infusion. Panel B shows the immunologic response of 2 patients with informative tetramers in the EBV-CTLs infused. The right panels show the frequency of tetramer-positive T cells on PBMCs collected before infusion. Left panels show that the frequency of the tetramer-positive T cells increased after CTL infusion.
Twelve patients received the CTLs, which had broadly comparable effects whether they were administered as a single dose or as multiple doses. Both regimens appeared safe and both induced a rise in EBV-responsive T cells in the circulation. No patient in either treatment group developed PTLD, and the patients suffering from overt PTLD showed a clinical response to single- and multiple-injection regimens. The infused CTLs therefore appear to be able to expand in vivo, to be able to traffic to and infiltrate tumor sites, and to control emergent disease. Nonetheless, the degree of expansion, length of persistence, and antiviral activity of the CTLs (measured by effects on EBV-DNA levels) were all less striking than equivalent doses of these cells in HSCT recipients. Thus, we observed only a 3- to 5-fold increase in the frequency of EBV-specific T cells within the first 6 weeks after infusion (measured by IFN-γ production or with tetramers) compared with a median of 32-fold expansion in HSCT recipients.9 The modest expansion and persistence observed in SOT recipients is likely a consequence in part of the immunosuppressive therapy, which continues in these patients during and after CTL infusion, since we have previously shown that immunosuppression can affect the proliferation and expansion of EBV-CTLs.18 It is also likely that the more limited expansion we observe after HSC transplantation can partly be attributed to an environment favoring lymphoid compartment expansion, such as exists after HSC transplantation or lymphodepleting chemotherapy.27 Of note, however, multidose administration of CTLs did increase persistence for up to 6 months, which indicates that this regimen may be preferable for a sustained response in individuals whose lymphoid compartment is replete.
In vivo antitumor activity of EBV-CTL therapy. Patient no. 3 received EBV-CTLs containing greater than 90% of CD3+CD4+ T cells. Eight weeks after the infusion, for an increase of liver enzymes, a biopsy was performed to exclude the occurrence of graft rejection (A). Immunohistochemistry analysis shows presence of B cells positive for LMP1 (top panels) and massive infiltration of CD4+ T cells, surrounding the EBV-positive areas (bottom panels), suggesting the specific homing of CTLs in the tumor area. (B) Biopsy at resolution: no LMP1-positive cells are detectable. Images were acquired with a Zeiss Axioskop microscope (Carl Zeiss, Gottingen, Germany) with a 20×/0.50 NA Neofluor objective lens. Cells were stained with hematoxylin (Mayer)–eosin. Images were photographed with a Spotmatic RT camera and processed with Spotmatic 3.4 PC/3.3.2 (Diagnostic Instruments, Sterling Heights, MI).
In vivo antitumor activity of EBV-CTL therapy. Patient no. 3 received EBV-CTLs containing greater than 90% of CD3+CD4+ T cells. Eight weeks after the infusion, for an increase of liver enzymes, a biopsy was performed to exclude the occurrence of graft rejection (A). Immunohistochemistry analysis shows presence of B cells positive for LMP1 (top panels) and massive infiltration of CD4+ T cells, surrounding the EBV-positive areas (bottom panels), suggesting the specific homing of CTLs in the tumor area. (B) Biopsy at resolution: no LMP1-positive cells are detectable. Images were acquired with a Zeiss Axioskop microscope (Carl Zeiss, Gottingen, Germany) with a 20×/0.50 NA Neofluor objective lens. Cells were stained with hematoxylin (Mayer)–eosin. Images were photographed with a Spotmatic RT camera and processed with Spotmatic 3.4 PC/3.3.2 (Diagnostic Instruments, Sterling Heights, MI).
Despite the limited CTL expansion, there was evidence of in vivo cytotoxic activity. There was a rise in plasma EBV-DNA, suggesting lysis of infected target cells, but only a variable reduction in the cell-associated EBV-DNA viremia, suggesting that lysis occurred only in a fraction of the infected cells. The apparently limited effects on circulating EBV-infected B cells are strikingly different from the apparently more complete effects at sites of frank PTLD. This difference may arise because the localized PTLD creates a proinflammatory cytokine environment that allows CTLs to overcome the medical immunosuppression. An alternative explanation for the inability of infused EBV-CTLs to reduce virus load in the periphery after SO transplantation, by contrast to findings in HSCT recipients, may be found in the circulating EBV-infected B cells in SOT recipients with high levels of viremia, which express only a limited array of poorly immunogenic EBV antigens such as LMP2 and EBNA1,28 whereas the EBV-PTLD cells express the more immunogenic EBV antigens as well.26
The approach we describe, although methodologically robust and apparently safe and effective, requires a specific autologous cell line to be manufactured for each patient. A simpler approach was suggested by Haque et al,12 who generated a bank of EBV-CTLs from healthy EBV-seropositive donors which were cryopreserved and subsequently infused in patients with PTLD based on the closest possible HLA match. They treated 8 patients with established PTLD after SO transplantation. The outcomes were promising, with 3 complete remissions and 1 partial response.12 However, these CTLs were cleared within 11 to 44 days, likely due to alloreactive recipient T cells, raising concerns about the durability of such responses.12 We suggest that the use of autologous T cells can be readily implemented in a cost-effective manner by careful selection of patients who would be likely to benefit from the approach. Our approach in this study led to a completion rate of only 34% (12/35), but based on our data, a more efficient manufacturing policy could be considered. We propose that when SOT patients first develop high EBV-DNA levels, then there should be a modest reduction in immunosuppression. If signs of rejection emerge or EBV-DNA levels continue to rise and/or overt PTLD develops (clinically or on imaging), then an autologous EBV-CTL line is initiated. As an interim measure, the patients are simultaneously and promptly treated with CD20 monoclonal antibody (rituximab), a well-tolerated and at least transiently effective therapy.29-31 During the period of monoclonal antibody (MAb) control, preparation of the EBV-CTLs is completed and the cells are administered.
We suggest that the treatment combination we propose, which is based not only on EBV disease cytoreduction but also on using CTLs to restore the immune response to the underlying oncogenic virus, will prove to be highly cost effective, since it should spare both the allograft and the patient.
Prepublished online as Blood First Edition Paper, July 11, 2006; DOI 10.1182/blood-2006-05-021782.
Supported by the General Clinical Research Center (GCRC) at Baylor College of Medicine (RR00188), the Methodist Foundation (B.S.), a Glaser Foundation fellowship (B.S.), a Doris Duke Distinguished Clinical Scientist Award (H.E.H.), a Doris Duke Clinical Scientist Development Award (S.G.), National Institutes of Health (NIH) DK74026 (J.A.G.), and NIH CA61384 (C.M.R.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful for the persistent and reliable support of Tatiana Gotsolva and April Durett of the Flow Cytometry Core Facility for FACS analyses. We thank the QA/QC group of the GMP facility in the Center for Cell and Gene Therapy at Baylor College of Medicine for excellent technical assistance. We thank Jaymee Scott and Lisa Bristow, from the Department of Surgery at Baylor College of Medicine, for taking care of the patients for this study. We also thank Dr Gianpietro Dotti (Center for Cell and Gene Therapy, Baylor College of Medicine) for helpful discussion.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal