Abstract
In this retrospective study, we analyzed the outcomes of 129 patients who underwent an allogeneic hematopoietic stem cell transplantation (allo-HSCT) and had a history of probable or proven invasive aspergillosis (IA), of whom 57 (44%) received a reduced-intensity conditioning (RIC). Overall, 27 patients with IA progressed after the allo-HSCT (cumulative incidence [CumInc] at 2 years, 22%). The variables that increased the 2-year CumInc of IA progression were (1) longer duration of neutropenia after transplantation; (2) advanced status of the underlying disease; and (3) less than 6 weeks from start of systemic anti-Aspergillus therapy and the allo-HSCT. In addition, (4) conventional myeloablative conditioning increased the risk of progression early after transplantation (before day 30) only, while 3 variables increased the risk beyond day 30 were (5) cytomegalovirus disease; (6) bone marrow or cord blood as source of stem cells; and (7) grades II to IV acute graft-versus-host disease (GVHD). A risk model for progression was generated, defined as low (0-1 risk factors, 6% incidence), intermediate (2-3 risk factors, 27% incidence), or high risk (≥ 3 risk factors, 72% incidence [P < .001]). These findings may help in the interpretation and design of future studies on secondary prophylaxis of IA after an allo-HSCT.
Introduction
Invasive aspergillosis (IA) is a common infectious complication during remission-induction and/or consolidation chemotherapy for aggressive hematologic malignancies, in particular in patients with acute leukemia and high-risk myelodysplastic syndrome. Many of these patients will subsequently be referred for an allogeneic hematopoietic stem cell transplantation (allo-HSCT), and due to ongoing improvements in supportive care and following the introduction of less toxic reduced-intensity conditioning (RIC) regimens, these transplantations are now also offered to older and more debilitated patients.1-8 Until a few years ago, IA was considered an almost absolute contraindication for an allo-HSCT due to the high risk of progression (or relapse) of IA after transplantation and the higher norelapse mortality rate (NRM). Indeed, tissue damage resulting from prior IA (mainly invasive pulmonary aspergillosis [IPA]) or its therapy and other common coexistent comorbidities translate into a higher NRM.5,9,10 In more recent years, 2 factors may have changed this attitude: (1) availability of new more effective and/or less toxic antifungal agents (lipid formulations of amphothericin B, voriconazole, and caspofungin); and (2) introduction of RICs, which have less early toxicity and fewer days of cytopenias.11-13 In fact, several case series report successful outcomes after RIC in patients with prior IA, although successful outcomes have also been obtained with conventional conditioning if supported by intensive medical and surgical care.10,14-19
However, very little data exist on factors that could predict adverse outcomes in patients with prior IA, although such data would be helpful for developing optimal management strategies for these patients. Only 2 studies with a patient sample size larger than 20 cases have been published to date.12,20 This paucity of data is not surprising, since it is still difficult to obtain a diagnosis of IA with a high level of certainty, and most patients with high-risk malignancies cannot have their allo-HSCT delayed for months in an effort to obtain a certain diagnosis. In addition, since the outcomes of allo-HSCT have improved worldwide in the late 1990s and early 2000s (especially with respect to lowering the NRM), data on more recent transplantation patients in multiple institutions would be desirable.
In this study, the Infectious Diseases Working Party of The European Group for Blood and Marrow Transplantation (IDWP-EBMT) sought to determine the outcomes of patients with a recent history of proven or probable IA who underwent allogeneic HSCT with myeloablative or reduced-intensity conditioning.
Patients and methods
Study design
A number of allo-HSCT centers (44) from 12 countries were willing to participate in this retrospective study. All centers agreed to complete an extensive case report form per eligible patient. Eligible patients were patients who received a first allo-HSCT from 1998 to 2004 and had been diagnosed before transplantation with probable or proven IA defined by the 2002 European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC-MSG) consensus guidelines.21 Patients with possible IA or non-Aspergillus mold infections were excluded from the study. A center-by-center effort was made to ensure that all consecutive patients were included, In addition, the 2 first authors reviewed all case report forms for completeness and consistency; if necessary, queries were sent to ensure data quality.
Definitions
The definitions were clearly specified in the protocol and in the case report forms. IA that occurred before allo-HSCT was defined as proven or probable as previously specified.21 Briefly, proven disease required histopathologic and microbiological documentation of IA from biopsied tissues. The infection was considered probable if the fungus was identified from culture of bronchoalveolar lavage or sputum (or ≥ 2 consecutive serum samples with a galactomannan index ≥ 0.8) together with suggestive clinical and radiologic signs and symptoms. The day of diagnosis of the fungal infection was the day on which the first positive test (radiologic and/or microbiological) was performed.
Response to antifungal therapy was reassessed immediately before transplantation using recently established criteria.22 Patients had a complete response (CR) if all clinical and radiologic signs and symptoms attributable to IA had disappeared, while partial response (PR) required a reduction in all lesions of more than 50%. The appearance of new lesions (in the same organ or in a new location) due to IA and an increase in established lesions were considered to be progression of the IA, while patients with a radiologic response lower than 50% were considered as having stable disease (SD).
Progression of the IA occurred in all patients as an increase of the lesions at the same anatomic location of the initial infection, while all 3 patients with breakthrough invasive fungal infections (IFIs) occurred in different sites and were caused by different fungal pathogens. Death was considered IA related when progression of the infection occurred immediately before death and/or when IA was found at autopsy.
Conventional myeloablative (CONV) conditioning included intravenous (IV) cyclophosphamide (120 mg/kg) plus total-body irradiation (TBI; ≥ 8 Gy) or cyclophosphamide (120 mg/kg) plus busulphan (16 mg/kg total dose by mouth or the equivalent IV dose), with or without other cytotoxic agents and/or antithymocyte globulin (ATG) or alemtuzumab. RIC included fludarabine plus intermediate doses of 1 or 2 alkylating agents or low-dose TBI (2-4 Gy), with or without ATG or alemtuzumab. Intermediate doses of alkylating agents consisted of busulphan (8-10 mg/kg orally), IV melphalan (80-140 mg/m2 ), IV cyclophosphamide (60 to 120 mg/kg), or IV thiotepa (5-10 mg/kg). Cytomegalovirus (CMV) disease, acute and chronic graft-versus-host disease (GVHD), and other transplantation outcomes were defined following accepted criteria.20
Patient characteristics at allo-HSCT
Characteristics of the 129 eligible patients are detailed in Table 1. The median age was 42 years (range, 5-72 years), and the underlying disease status was advanced in 45% of the patients. Although peripheral blood stem cell transplantation (PBSCT) from a human leukocyte antigen (HLA)–identical sibling was the most common type of allo-HSCT, there was also a high percentage (45%) of alternative donor transplantations. Nearly half of the patients (57; 44%) received RIC. This latter group differed from the CONV group in several characteristics, including older age, higher proportion of PBSCTs, more use of antilymphocyte antibodies as part of the conditioning, and less use of the “calcineurin inhibitor plus methotrexate regimen” for the prevention of GVHD. Informed consent was obtained locally in accordance with the principles laid out in the Declaration of Helsinki and according to the local and national approvals applicable according to the specific trial followed by each center.
Patient characteristics and overall transplantation outcome
. | All patients . | RIC . | Conventional . | P . |
|---|---|---|---|---|
| No. patients | 129 | 57 | 72 | |
| Median age, y (range) | 42 (5-72) | 50 (5-72) | 37 (8-64) | .02 |
| Male sex, no. (%) | 78 (60) | 33 (58) | 45 (62) | |
| Female donor, no. (%) | 43 (33) | 21 (37) | 22 (31) | |
| Patient CMV seropositive (IgG), no. (%) | 79 (61) | 35 (61) | 44 (61) | |
| Seronegative donors, no. (%) | 29 (33) | 17 (30) | 12 (17) | |
| Underlying disease, no. (%) | ||||
| Acute leukemia or myelodysplasia | 107 (83) | 46 (81) | 61 (85) | |
| Chronic myelogenous leukemia | 5 (4) | 3 (5) | 2 (3) | |
| Lymphoma | 8 (6) | 6 (10) | 2 (3) | |
| Multiple myeloma or CLL | 3 (2) | 1 (2) | 2 (3) | |
| Other | 6 (5) | 1 (2) | 5 (7) | |
| Disease status at transplantation, no. (%)* | ||||
| Early | 44 (34) | 20 (35) | 24 (33) | |
| Advanced | 85 (66) | 37 (65) | 48 (67) | |
| Prior autologous HSCT, no. (%) | 10 (8) | 6 (11) | 4 (6) | |
| Donor type, no. (%) | ||||
| HLA-identical sibling | 71 (55) | 28 (50) | 43 (60) | |
| Alternative (VUD/family mismatched) | 58 | 29 | 29 | |
| Stem cell source, no. (%) | ||||
| Peripheral blood | 99 (77) | 49 (86) | 50 (69) | .03 |
| Bone marrow | 28 | 8 | 20 | |
| Cord blood | 2 | — | 2 | |
| Transplantation year, no. (%) | ||||
| 1998-2000 | 32 (25) | 11 (19) | 21 (29) | |
| 2001-2004 | 97 (75) | 46 (81) | 51 (71) | |
| ATG or alemtuzumab in conditioning, no. (%) | 60 (47) | 34 (60) | 26 (36) | < .01 |
| Ex vivo T-cell depletion, no. (%) | 22 (17) | 16 (28) | 6 (8) | .04 |
| Conditioning regimen, no. (%) | ||||
| TBI-based conventional myeloablative therapy | — | — | 49 (68) | |
| Chemotherapy-only conventional myeloablative therapy | — | — | 23 (32) | |
| Reduced-intensity therapy | ||||
| Alkylating agent(s) + fludarabine | — | 48 (84) | — | |
| Low-dose TBI (≤ 4 Gy) + fludarabine | — | 9 (16) | — | |
| GVHD prophylaxis, no. (%) | .04 | |||
| CsA ± steroids ± ATG | 36 (28) | 22 (39) | 14 (19) | |
| CsA or Tacro + MTX ± ATG | 77 (60) | 21 (37) | 56 (78) | |
| CsA or Tacro + MMF ± ATG | 16 (12) | 14 (25) | 2 (3) | |
| Median d of neutropenia (range)† | 18 (1-123) | 10 (1-123) | 19 (8-93) | .03 |
| Less than 7 d of neutropenia, no. (%) | 20 (16) | 14 (25) | 6 (8) | .02 |
| Less than 21 d of neutropenia, no. (%) | 81 (63) | 41 (72) | 40 (56) | |
| Stable donor engraftment, no. (%) | 118 (91) | 56 (97) | 62 (87) | .04 |
| No. developing grades II-IV aGVHD (% CumInc, 95% CI) | 53 (36, 28-44) | 17 (26, 14-38) | 36 (44, 32-56) | .03 |
| No. developed cGVHD (% CumInc, 95% CI) | 45 (30, 18-42) | 24 (34, 20-48) | 21 (26, 16-36) | |
| 2-y nonrelapse mortality, % CumInc (95% CI) | 30 (19-39) | 29 (18-48) | 30 (19-39) | |
| 2-y disease relapse, % CumInc (95% CI) | 33 (19-45) | 32 (13-45) | 35 (20-50) | |
| Overall survival, % CumInc (95% CI) | 42 (30-50) | 42 (24-52) | 42 (25-53) | |
| Median d follow-up (range) | 287 (2-1414) | 253 (2-1326) | 365 (7-1414) | .1 |
. | All patients . | RIC . | Conventional . | P . |
|---|---|---|---|---|
| No. patients | 129 | 57 | 72 | |
| Median age, y (range) | 42 (5-72) | 50 (5-72) | 37 (8-64) | .02 |
| Male sex, no. (%) | 78 (60) | 33 (58) | 45 (62) | |
| Female donor, no. (%) | 43 (33) | 21 (37) | 22 (31) | |
| Patient CMV seropositive (IgG), no. (%) | 79 (61) | 35 (61) | 44 (61) | |
| Seronegative donors, no. (%) | 29 (33) | 17 (30) | 12 (17) | |
| Underlying disease, no. (%) | ||||
| Acute leukemia or myelodysplasia | 107 (83) | 46 (81) | 61 (85) | |
| Chronic myelogenous leukemia | 5 (4) | 3 (5) | 2 (3) | |
| Lymphoma | 8 (6) | 6 (10) | 2 (3) | |
| Multiple myeloma or CLL | 3 (2) | 1 (2) | 2 (3) | |
| Other | 6 (5) | 1 (2) | 5 (7) | |
| Disease status at transplantation, no. (%)* | ||||
| Early | 44 (34) | 20 (35) | 24 (33) | |
| Advanced | 85 (66) | 37 (65) | 48 (67) | |
| Prior autologous HSCT, no. (%) | 10 (8) | 6 (11) | 4 (6) | |
| Donor type, no. (%) | ||||
| HLA-identical sibling | 71 (55) | 28 (50) | 43 (60) | |
| Alternative (VUD/family mismatched) | 58 | 29 | 29 | |
| Stem cell source, no. (%) | ||||
| Peripheral blood | 99 (77) | 49 (86) | 50 (69) | .03 |
| Bone marrow | 28 | 8 | 20 | |
| Cord blood | 2 | — | 2 | |
| Transplantation year, no. (%) | ||||
| 1998-2000 | 32 (25) | 11 (19) | 21 (29) | |
| 2001-2004 | 97 (75) | 46 (81) | 51 (71) | |
| ATG or alemtuzumab in conditioning, no. (%) | 60 (47) | 34 (60) | 26 (36) | < .01 |
| Ex vivo T-cell depletion, no. (%) | 22 (17) | 16 (28) | 6 (8) | .04 |
| Conditioning regimen, no. (%) | ||||
| TBI-based conventional myeloablative therapy | — | — | 49 (68) | |
| Chemotherapy-only conventional myeloablative therapy | — | — | 23 (32) | |
| Reduced-intensity therapy | ||||
| Alkylating agent(s) + fludarabine | — | 48 (84) | — | |
| Low-dose TBI (≤ 4 Gy) + fludarabine | — | 9 (16) | — | |
| GVHD prophylaxis, no. (%) | .04 | |||
| CsA ± steroids ± ATG | 36 (28) | 22 (39) | 14 (19) | |
| CsA or Tacro + MTX ± ATG | 77 (60) | 21 (37) | 56 (78) | |
| CsA or Tacro + MMF ± ATG | 16 (12) | 14 (25) | 2 (3) | |
| Median d of neutropenia (range)† | 18 (1-123) | 10 (1-123) | 19 (8-93) | .03 |
| Less than 7 d of neutropenia, no. (%) | 20 (16) | 14 (25) | 6 (8) | .02 |
| Less than 21 d of neutropenia, no. (%) | 81 (63) | 41 (72) | 40 (56) | |
| Stable donor engraftment, no. (%) | 118 (91) | 56 (97) | 62 (87) | .04 |
| No. developing grades II-IV aGVHD (% CumInc, 95% CI) | 53 (36, 28-44) | 17 (26, 14-38) | 36 (44, 32-56) | .03 |
| No. developed cGVHD (% CumInc, 95% CI) | 45 (30, 18-42) | 24 (34, 20-48) | 21 (26, 16-36) | |
| 2-y nonrelapse mortality, % CumInc (95% CI) | 30 (19-39) | 29 (18-48) | 30 (19-39) | |
| 2-y disease relapse, % CumInc (95% CI) | 33 (19-45) | 32 (13-45) | 35 (20-50) | |
| Overall survival, % CumInc (95% CI) | 42 (30-50) | 42 (24-52) | 42 (25-53) | |
| Median d follow-up (range) | 287 (2-1414) | 253 (2-1326) | 365 (7-1414) | .1 |
CLL indicates chronic lymphocytic leukemia; VUD, volunteer unrelated donor; CsA, cyclosporine A; MTX, methotrexate; MMF, mycophenolate mofetil; Tacro, tacrolimus; and —, not applicable.
Early indicates acute leukemia and myelodysplastic syndrome in first complete remission after chemotherapy (< 5% blasts), previously untreated low-risk MDS, and chronic myeloid leukemia in first chronic phase; advanced, malignancies in a phase beyond the early phase, and multiple myeloma, CLL, and non-Hodgkin lymphoma, Hodgkin disease, and solid tumors.
Neutropenia is defined as < 0.5 × 109 cells/L.
Treatment of IA before transplantation
Characteristics of IA before transplantation are detailed in Table 2. The median duration of antifungal therapy (AFT) was 16.4 weeks (range, 1-50 weeks). Nearly all patients (116; 90%) received more than one drug, either in combination or sequentially. Antifungal drugs included conventional amphotericin B (c-AmB) in 59 patients, itraconazole in 51 patients, liposomal AmB (L-AmB) in 41 patients, caspofungin in 35 patients, voriconazole in 30 patients, and amphotericin B lipid complex in 25 patients. A smaller number of patients (28; 22%) also underwent surgery, mostly pulmonary lobectomy. A number of patients (49; 38%) were classified as proven IA; the remainder met the criteria of probable IA. Despite AFT, a CR or PR was not obtained in 21 patients (16%), as shown in Table 2.
Characteristics and posttransplantation outcomes of invasive aspergillosis
. | All patients . | RIC . | Conventional . | P* . |
|---|---|---|---|---|
| No. patients | 129 | 57 | 72 | |
| Classification by the EORTC-MSG criteria, no. (%) | ||||
| Proven | 49 (38) | 12 (21) | 37 (51) | .01 |
| Probable | 80 | 45 | 35 | |
| Aspergillus species involved, no. (%) | ||||
| A fumigatus | 40 (31) | 11 (19) | 29 (40) | |
| A flavus | 3 (2) | 1 (2) | 2 (3) | |
| A terreus | 2 (1) | 1 (2) | 1 (1) | |
| A niger | 3 (2) | 2 (4) | 1 (1) | |
| Nonspeciated species | 38 (29) | 20 (35) | 18 (25) | |
| GM positive† | 44 (34) | 23 (40) | 21 (29) | |
| Sites involved, no. (%) | ||||
| Pulmonary with or without other | 117 (91) | 53 (93) | 64 (89) | |
| Extrapulmonary | 12 | 4 | 8 | |
| Time interval from start TxIA-Allo-HSCT, median d (range) | 101 (9-378) | 98 (58-371) | 103 (9-378) | |
| Less than 6 wk, no. (%) | 18 (14) | 9 (16) | 9 (11) | |
| 6 wk to 3 mo, no. (%) | 38 (29) | 15 (26) | 23 (32) | |
| 3 to 6 mo, no. (%) | 43 (33) | 19 (33) | 24 (33) | |
| 6 to 12 mo, no. (%) | 30 (23) | 14 (25) | 16 (22) | |
| Status of IA at Allo-HSCT, no. (%) | .06 | |||
| Complete remission | 58 (45) | 21 (37) | 37 (51) | |
| Partial remission | 50 (39) | 29 (51) | 21 (29) | |
| Stable disease | 17 (13) | 5 (9) | 12 (17) | |
| Progression | 4 (3) | 2 (3) | 2 (3) | |
| Surgical resection before allo-HSCT, no. (%) | 28 (22) | 4 (7) | 24 (33) | < .01 |
| No. GM positive (serum index ≥ 0.8 × 2 samples) at allo-HSCT/no. tested (%) | 5/53 (9) | 4/23 (17) | 1/30 (3) | .02 |
| New (breakthrough) IFI after allo-HSCT, no. (%)‡ | 3 (2) | 1 (2) | 2 (3) | |
| Final response of IA after allo-HSCT, no. (%) | ||||
| Stable disease | 58 (45) | 24 (42) | 34 (47) | |
| Improvement after transplantation | 44 (34) | 20 (35) | 24 (33) | |
| Progression | 27 (21) | 13 (23) | 14 (19) | |
| Incidence of progression of IA, no. (95% CI) | ||||
| 1 mo | 10 (2-24) | 7 (1-12) | 15 (5-24) | |
| 6 mo | 15 (8-22) | 12 (3-21) | 16 (7-39) | |
| 2 y | 22 (14-30) | 22 (8-32) | 21 (9-33) | |
| Response to salvage AFT of IA that progressed after allo-HSCT, no. (%) | 6/27 (22) | 5/13 (39) | 1/14 (7) | .08 |
| Deaths related to IA, no. (CumInc)§ | 21 (14) | 6 (18) | 15 (18) | .16* |
| Deaths related to IA/total deaths (%)§ | 21/73 (29) | 6/31 (19) | 15/42 (36) | .1 |
| Death related to any IFI (CumInc)§ | 24 (15) | 7 (9) | 17 (23) | .11* |
. | All patients . | RIC . | Conventional . | P* . |
|---|---|---|---|---|
| No. patients | 129 | 57 | 72 | |
| Classification by the EORTC-MSG criteria, no. (%) | ||||
| Proven | 49 (38) | 12 (21) | 37 (51) | .01 |
| Probable | 80 | 45 | 35 | |
| Aspergillus species involved, no. (%) | ||||
| A fumigatus | 40 (31) | 11 (19) | 29 (40) | |
| A flavus | 3 (2) | 1 (2) | 2 (3) | |
| A terreus | 2 (1) | 1 (2) | 1 (1) | |
| A niger | 3 (2) | 2 (4) | 1 (1) | |
| Nonspeciated species | 38 (29) | 20 (35) | 18 (25) | |
| GM positive† | 44 (34) | 23 (40) | 21 (29) | |
| Sites involved, no. (%) | ||||
| Pulmonary with or without other | 117 (91) | 53 (93) | 64 (89) | |
| Extrapulmonary | 12 | 4 | 8 | |
| Time interval from start TxIA-Allo-HSCT, median d (range) | 101 (9-378) | 98 (58-371) | 103 (9-378) | |
| Less than 6 wk, no. (%) | 18 (14) | 9 (16) | 9 (11) | |
| 6 wk to 3 mo, no. (%) | 38 (29) | 15 (26) | 23 (32) | |
| 3 to 6 mo, no. (%) | 43 (33) | 19 (33) | 24 (33) | |
| 6 to 12 mo, no. (%) | 30 (23) | 14 (25) | 16 (22) | |
| Status of IA at Allo-HSCT, no. (%) | .06 | |||
| Complete remission | 58 (45) | 21 (37) | 37 (51) | |
| Partial remission | 50 (39) | 29 (51) | 21 (29) | |
| Stable disease | 17 (13) | 5 (9) | 12 (17) | |
| Progression | 4 (3) | 2 (3) | 2 (3) | |
| Surgical resection before allo-HSCT, no. (%) | 28 (22) | 4 (7) | 24 (33) | < .01 |
| No. GM positive (serum index ≥ 0.8 × 2 samples) at allo-HSCT/no. tested (%) | 5/53 (9) | 4/23 (17) | 1/30 (3) | .02 |
| New (breakthrough) IFI after allo-HSCT, no. (%)‡ | 3 (2) | 1 (2) | 2 (3) | |
| Final response of IA after allo-HSCT, no. (%) | ||||
| Stable disease | 58 (45) | 24 (42) | 34 (47) | |
| Improvement after transplantation | 44 (34) | 20 (35) | 24 (33) | |
| Progression | 27 (21) | 13 (23) | 14 (19) | |
| Incidence of progression of IA, no. (95% CI) | ||||
| 1 mo | 10 (2-24) | 7 (1-12) | 15 (5-24) | |
| 6 mo | 15 (8-22) | 12 (3-21) | 16 (7-39) | |
| 2 y | 22 (14-30) | 22 (8-32) | 21 (9-33) | |
| Response to salvage AFT of IA that progressed after allo-HSCT, no. (%) | 6/27 (22) | 5/13 (39) | 1/14 (7) | .08 |
| Deaths related to IA, no. (CumInc)§ | 21 (14) | 6 (18) | 15 (18) | .16* |
| Deaths related to IA/total deaths (%)§ | 21/73 (29) | 6/31 (19) | 15/42 (36) | .1 |
| Death related to any IFI (CumInc)§ | 24 (15) | 7 (9) | 17 (23) | .11* |
TxIA-allo-HSCT indicates time interval from start of antifungal therapy for IA and allo-HSCT.
All P values were calculated with the chi-square statistic or Fisher exact test except for comparisons of cumulative incidence estimates, which were compared with a univariate Cox regression model (detailed in “Statistical analysis”).
GM positivity in serum (index ≥ 0.8 twice) without culture isolation of the causative species. An additional 24 patients had positive GM in serum and isolation of the causative Aspergillus spp, and 1 patient had 2 species isolated from the same sample (A fumigatus and A niger).
All breakthrough infections occurred under systemic antifungal secondary prophylaxis. One patient on voriconazole developed proven disseminated fusariosis, 1 on itraconazole developed a central nervous system infection from an unidentified fungus with pseudohyphae, and 1 patient on itraconazole developed proven pulmonary zygomycosis.
All outcomes analyzed at 2 years after transplantation.
Statistical analysis
The major goal of this study was to identify risk factors for progression of IAafter allo-HSCT. The incidence of progression of IA and NRM were estimated by cumulative incidence starting on the day of transplantation, with death and relapse of underlying malignancy as competing risks, while the probability of overall survival (OS) was estimated using the Kaplan-Meier product-limit estimate with standard methods.23,24 Other posttransplantation outcomes that were calculated using cumulative incidence estimates were acute and chronic GVHD, incidence of death due to IA and death due to any IFI. Univariate analyses of the association of various clinical risk factors with the hazard ratio of developing progression of IA were calculated using univariate Cox regression models, while the log-rank test was used for OS. Multivariate analyses were performed by Cox proportional hazards regression, with inclusion of variables with a P value less than .1 in the prior univariate testing. The assumption of proportional hazards over time was tested for all explanatory covariates using a time-dependent covariate. Several variables (CMV disease, intensity of the conditioning, stem cell source, and acute GVHD grades II-IV) had a time-varying effect on the hazard ratio (or “risk”) of progression of IA, and thus the risk factor analysis was divided into early and late progression at an optimal time point estimated by visual analysis of the cumulative incidence curves (before and after day 30, respectively). Posttransplantation variables (positive galactomannan [GM] in serum, acute and chronic GVHD, CMV disease, and duration of neutropenia) were analyzed as time-dependent covariates.
Quantitative variables that were found to have an impact on any outcome were reanalyzed as categoric variables. For categoric variables, the chi-square statistic or Fisher exact test were used to establish differences in their distribution; the Mann-Whitney U test was used to compare continuous variables. Tests of significance were 2-sided, with a significance P level of .05 or less. Variables analyzed for their impact on progression of IA are shown in detail in Table 3. All statistical analyses were performed using SPSS version 13.0 (SPSS, Chicago, IL), with the exception of the cumulative incidence analyses, which were carried out with NCSS 2004 (Number Cruncher Statistical System, Kaysville, UT).
Univariate and multivariate analysis of risk factors for progression of prior IA after transplantation
. | . | . | Progression < d 30 . | . | . | Progression after d 30 . | . | . | Overall follow-up . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Multivariate . | . | . | Multivariate . | . | . | Multivariate . | . | ||||||
. | No. patients . | 2-y incidence of IA progression (95% CI) . | Univariate P . | P . | HR (95% CI) . | Univariate P . | P . | HR (95% CI) . | Univariate P . | P . | HR (95% CI) . | ||||||
| Impact during the entire posttransplantaton period | |||||||||||||||||
| Duration of neutropenia*† | — | < .001 | < .01 | 10 (4-67)‡ | .01 | .01 | 10 (2.6-40) | < .001 | < .001 | 10.6 (5.4-37) | |||||||
| Status of the underlying disease | .07 | .3 | .01 | .15 | < .01 | .01 | 7 (1.6-30) | ||||||||||
| Not early | 85 | 30 (16-43) | |||||||||||||||
| Early | 44 | 5 (-1-11) | |||||||||||||||
| Wk between start TxIA-Allo-HSCT†§ | |||||||||||||||||
| Less than 6 | 18 | 34 (9-58) | .04 | .02 | 4.6 (1.3-16) | .06 | .11 | < .01 | .01 | 3.6 (1.4-9.4) | |||||||
| At least 6 | 111 | 16 (7-25) | |||||||||||||||
| Response status of the IA at HSCT§ | |||||||||||||||||
| SD or progression | 21 | 32 (10-54) | .1 | NI§ | NI§ | .04 | NI§ | NI§ | .01§ | — | —§ | ||||||
| CR or PR | 108 | 17 (8-26) | |||||||||||||||
| Pharmacologic prophylaxis for GVHD | .03 | .11 | — | .04 | .2 | .03 | |||||||||||
| CsA or Tacro + MTX | 76 | 16 (9-30) | .08 | — | |||||||||||||
| Other non-MTX | 53 | 26 (16-36) | |||||||||||||||
| Impact during the early posttransplantation period (< d 30)∥ | |||||||||||||||||
| Type of conditioning | .02 | .054 | 3.4 (0.98-12) | .5 | — | — | .4∥ | — | — | ||||||||
| CONV | 72 | 21 (9-33) | |||||||||||||||
| RIC | 57 | 22 (8-32) | |||||||||||||||
| Impact during the late posttransplantation period (> d 30)∥ | |||||||||||||||||
| CMV disease* | NA | NA | NA | < .001 | |||||||||||||
| Yes | 12 | 40 (10-70) | .02 | 4.2 (1.4-17) | .02∥ | .04 | 3.7 (1.3-11) | ||||||||||
| No | 117 | 15 (7-23) | |||||||||||||||
| Stem cell source | 21 (8-34) | .4 | — | — | < .001 | ||||||||||||
| BMT or CBT | 30 | 15 (3-27) | .01 | 9.8 (9-99) | .4∥ | — | — | ||||||||||
| PBSC | 99 | ||||||||||||||||
| Acute GVHD ≥ grade II that required HD steroids (> 1 wk) and/or ATG* | NA | NA | NA | .01 | .04 | 10 (1.7-29) | .04 | .3 | — | ||||||||
| Yes | 53 | 31 (15-47) | |||||||||||||||
| No | 76 | 16 (6-26) | |||||||||||||||
| Variables with no impact | |||||||||||||||||
| Patient age† | — | .96 | — | — | .93 | — | — | .96 | — | — | |||||||
| Donor type | .9 | — | — | .93 | — | — | .9 | — | — | ||||||||
| Alternative donor | 58 | 22 (8-36) | |||||||||||||||
| HLA-identical sibling | 71 | 18 (7-29) | |||||||||||||||
| Type of IA | .9 | — | — | .9 | — | — | .9 | — | — | ||||||||
| Proven | 49 | 22 (9-35) | |||||||||||||||
| Probable | 80 | 18 (8-28) | |||||||||||||||
| Surgical resection before allo-HSCT | .4 | — | — | .3 | — | — | .35 | — | — | ||||||||
| Yes | 28 | 18 (0-36) | |||||||||||||||
| No | 101 | 23 (13-333) | |||||||||||||||
| Chronic GVHD* | NA | NA | NA | .8 | — | — | .8 | — | — | ||||||||
| Yes | 45 | 24 (9-39) | |||||||||||||||
| No | 84 | 20 (9-31) | |||||||||||||||
| ATG or alemtuzumab in conditioning | .4 | — | — | .5 | — | — | .6 | — | — | ||||||||
| Yes | 60 | 23 (5-40) | |||||||||||||||
| No | 69 | 17 (6-28) | |||||||||||||||
| Ex vivo T-cell depletion | .8 | — | — | .7 | — | — | .8 | — | — | ||||||||
| Yes | 22 | 22 (6-38) | |||||||||||||||
| No | 107 | 26 (9-27) | |||||||||||||||
| 2 or more post-HSCT serum samples with positive GM, index ≥ 0.8 (%)* | .03 | NT§ | NT§ | .2 | — | — | .002† | NT | NT¶ | ||||||||
| No | 43 | 2/43 (4.7) | |||||||||||||||
| Yes | 24 | 11/24 (46) | |||||||||||||||
| Not done¶ | 62 | 14/62 (22.6) | |||||||||||||||
. | . | . | Progression < d 30 . | . | . | Progression after d 30 . | . | . | Overall follow-up . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | Multivariate . | . | . | Multivariate . | . | . | Multivariate . | . | ||||||
. | No. patients . | 2-y incidence of IA progression (95% CI) . | Univariate P . | P . | HR (95% CI) . | Univariate P . | P . | HR (95% CI) . | Univariate P . | P . | HR (95% CI) . | ||||||
| Impact during the entire posttransplantaton period | |||||||||||||||||
| Duration of neutropenia*† | — | < .001 | < .01 | 10 (4-67)‡ | .01 | .01 | 10 (2.6-40) | < .001 | < .001 | 10.6 (5.4-37) | |||||||
| Status of the underlying disease | .07 | .3 | .01 | .15 | < .01 | .01 | 7 (1.6-30) | ||||||||||
| Not early | 85 | 30 (16-43) | |||||||||||||||
| Early | 44 | 5 (-1-11) | |||||||||||||||
| Wk between start TxIA-Allo-HSCT†§ | |||||||||||||||||
| Less than 6 | 18 | 34 (9-58) | .04 | .02 | 4.6 (1.3-16) | .06 | .11 | < .01 | .01 | 3.6 (1.4-9.4) | |||||||
| At least 6 | 111 | 16 (7-25) | |||||||||||||||
| Response status of the IA at HSCT§ | |||||||||||||||||
| SD or progression | 21 | 32 (10-54) | .1 | NI§ | NI§ | .04 | NI§ | NI§ | .01§ | — | —§ | ||||||
| CR or PR | 108 | 17 (8-26) | |||||||||||||||
| Pharmacologic prophylaxis for GVHD | .03 | .11 | — | .04 | .2 | .03 | |||||||||||
| CsA or Tacro + MTX | 76 | 16 (9-30) | .08 | — | |||||||||||||
| Other non-MTX | 53 | 26 (16-36) | |||||||||||||||
| Impact during the early posttransplantation period (< d 30)∥ | |||||||||||||||||
| Type of conditioning | .02 | .054 | 3.4 (0.98-12) | .5 | — | — | .4∥ | — | — | ||||||||
| CONV | 72 | 21 (9-33) | |||||||||||||||
| RIC | 57 | 22 (8-32) | |||||||||||||||
| Impact during the late posttransplantation period (> d 30)∥ | |||||||||||||||||
| CMV disease* | NA | NA | NA | < .001 | |||||||||||||
| Yes | 12 | 40 (10-70) | .02 | 4.2 (1.4-17) | .02∥ | .04 | 3.7 (1.3-11) | ||||||||||
| No | 117 | 15 (7-23) | |||||||||||||||
| Stem cell source | 21 (8-34) | .4 | — | — | < .001 | ||||||||||||
| BMT or CBT | 30 | 15 (3-27) | .01 | 9.8 (9-99) | .4∥ | — | — | ||||||||||
| PBSC | 99 | ||||||||||||||||
| Acute GVHD ≥ grade II that required HD steroids (> 1 wk) and/or ATG* | NA | NA | NA | .01 | .04 | 10 (1.7-29) | .04 | .3 | — | ||||||||
| Yes | 53 | 31 (15-47) | |||||||||||||||
| No | 76 | 16 (6-26) | |||||||||||||||
| Variables with no impact | |||||||||||||||||
| Patient age† | — | .96 | — | — | .93 | — | — | .96 | — | — | |||||||
| Donor type | .9 | — | — | .93 | — | — | .9 | — | — | ||||||||
| Alternative donor | 58 | 22 (8-36) | |||||||||||||||
| HLA-identical sibling | 71 | 18 (7-29) | |||||||||||||||
| Type of IA | .9 | — | — | .9 | — | — | .9 | — | — | ||||||||
| Proven | 49 | 22 (9-35) | |||||||||||||||
| Probable | 80 | 18 (8-28) | |||||||||||||||
| Surgical resection before allo-HSCT | .4 | — | — | .3 | — | — | .35 | — | — | ||||||||
| Yes | 28 | 18 (0-36) | |||||||||||||||
| No | 101 | 23 (13-333) | |||||||||||||||
| Chronic GVHD* | NA | NA | NA | .8 | — | — | .8 | — | — | ||||||||
| Yes | 45 | 24 (9-39) | |||||||||||||||
| No | 84 | 20 (9-31) | |||||||||||||||
| ATG or alemtuzumab in conditioning | .4 | — | — | .5 | — | — | .6 | — | — | ||||||||
| Yes | 60 | 23 (5-40) | |||||||||||||||
| No | 69 | 17 (6-28) | |||||||||||||||
| Ex vivo T-cell depletion | .8 | — | — | .7 | — | — | .8 | — | — | ||||||||
| Yes | 22 | 22 (6-38) | |||||||||||||||
| No | 107 | 26 (9-27) | |||||||||||||||
| 2 or more post-HSCT serum samples with positive GM, index ≥ 0.8 (%)* | .03 | NT§ | NT§ | .2 | — | — | .002† | NT | NT¶ | ||||||||
| No | 43 | 2/43 (4.7) | |||||||||||||||
| Yes | 24 | 11/24 (46) | |||||||||||||||
| Not done¶ | 62 | 14/62 (22.6) | |||||||||||||||
Data for progression before day 30 reflect 13 patients out of 129 evaluable patients; for progression after day 30, data reflect 14 patients out of 107 evaluable. Overall follow-up data are for 27 patients.
NA indicates not applicable, since no patient developed this outcome variable before day 30 after transplantation; HD (high-dose) steroids, prednisone ≥ 2 mg/kg; TxIA-Allo-HSCT, interval from start of AFT for IA and allo-HSCT; NT, not tested (see “Statistical analysis” and “Results” for details); NI, not included; —, not applicable (since P > .01).
Time-dependent variables.
Quantitative variables that were found to have an impact on any outcome were reanalyzed as categorical variables.
The HR shown refers to risk increase with every 5-day delay in the time for recovery of the absolute neutrophil count.
These 2 variables show collinearity, and for multivariate analysis the variable used was < 6 weeks from start of treatment of IA and the Allo-HSCT, because it showed a higher hazard ratio in univariate Cox regression and it is a more objectively measurable variable than the response of the IA at the time of transplantation.
These variables had a nonproportional hazard ratio over time of their impact on the risk of progression of the IA, which differed before and after day + 30 post-HSCT (see “Statistical analysis”).
Since only 67 patients (52%) were screened in the immediate posttransplantation period with serum GM performed at least twice weekly, this variable was not included in the multivariate analysis.
Center effect exclusion by sensitivity analysis
To assess the possibility of a center effect in the risk of progression of IA, we analyzed the progression rates from centers according to the number of patients reported. Five centers reported more than 10 evaluable patients (11-16 each), 3 centers had 6 to 10 patients, and 15 centers had 1 to 5 patients. No differences in progression rates were seen (chi-square test for a trend, P = .85).
Results
Incidence and risk factors for progression of IA after transplantation
IA progressed in 27 patients (21%) after transplantation. The incidence of progression at 2 years was 22% (95% confidence interval [CI], 14%-30%). Three additional patients developed a new breakthrough proven or probable IFI (Table 2). The incidence of progression of IA was similar in RIC and CONV recipients, although there was a trend toward a higher IA- and IFI-related mortality in the CONV group, as detailed in Table 2. Since 22 different strategies (specific drug[s] used, either in combination or sequentially, and changes in the route of administration) were used for secondary antifungal prophylaxis during the conditioning and posttransplantation follow-up, it is very difficult to identify differences between specific strategies. However, since voriconazole is considered the first-line drug for treatment of IA,22 we analyzed the impact of its use as initial secondary prophylaxis in the 31 patients who received this drug as monotherapy in the immediate post-HSCT period. The specific drugs used as first-line prophylaxis and after progression of the IA are shown in Tables 3 and 5.
Response to a change in the AFT used in patients who had progression of the IA after transplantation
. | No. patients treated as salvage AFT* . | No. patients who responded to salvage AFT (%) . |
|---|---|---|
| Salvage AFT given for IA after allo-HSCT | ||
| Azoles (itraconazole/voriconazole) | 12 (8/4) | 3 (25) |
| AmB formulations (c-AmB/ABLC/L-AmB) | 9 (2/3/4) | 3 (33) |
| Caspofungin | 4 | 2 (50) |
| No salvage AFT | 10 | 1 (10)† |
| All patients at risk | 27‡ | 6 (22) |
. | No. patients treated as salvage AFT* . | No. patients who responded to salvage AFT (%) . |
|---|---|---|
| Salvage AFT given for IA after allo-HSCT | ||
| Azoles (itraconazole/voriconazole) | 12 (8/4) | 3 (25) |
| AmB formulations (c-AmB/ABLC/L-AmB) | 9 (2/3/4) | 3 (33) |
| Caspofungin | 4 | 2 (50) |
| No salvage AFT | 10 | 1 (10)† |
| All patients at risk | 27‡ | 6 (22) |
AmB indicates amphotericin B; and ABLC, AmB lipid complex.
Salvage antifungal therapy used after progression of IA after HSCT.
One patient was not given salvage AFT for progression of IA because his leukemia recurred shortly thereafter. Nevertheless, IA decreased more than 50% within 3 weeks.
In 8 (30%) of 27 patients, more than 1 drug was used in combination.
Patients in PR or CR from IA at transplantation were less likely to progress regardless of the type of secondary prophylaxis used (17% vs 32% for those not in PR/CR, P = .012). In addition, patients who were failing to respond to AFT at transplantation and who subsequently progressed did not respond to salvage AFT (0% vs 32% for those who progressed but were in CR/PR at transplantation). Again, no specific AFT regimen was more likely to avoid progression or to obtain a response as salvage therapy. Pretransplantation surgical resection had no influence on any outcome analyzed (details not shown).
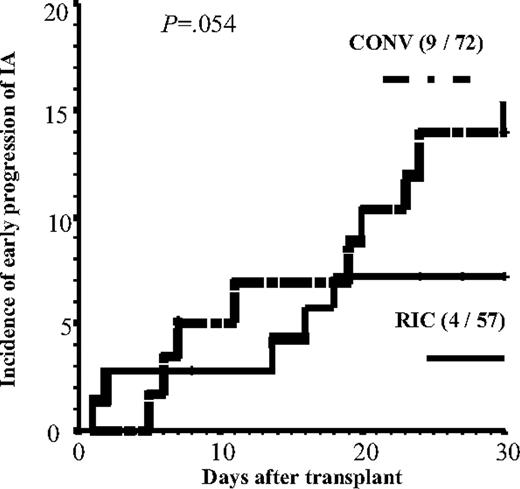
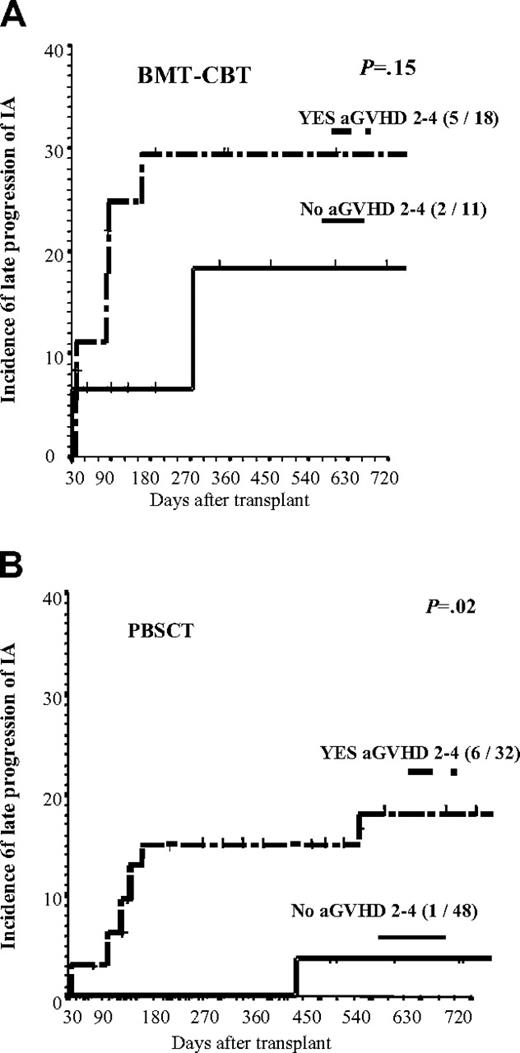
Four variables (CMV disease, intensity of the conditioning, stem cell source, and acute GVHD grades II-IV) had a time-varying effect on the hazard ratio (HR) of progression of IA (Table 4). In multivariate analysis, early progression (before day 30) of IA was more common in recipients of a CONV conditioning (7% vs 15% 1-month incidence in RIC vs CONV groups; Figure 1; P = .054). Three variables increased the risk of late progression (after day 30): CMV disease, bone marrow (BM)/cord blood (CB) as source of stem cells and acute GVHD (aGVHD) grades II to IV. Figure 2 shows the incidence of late progression of IA (after day 30) in patients according to type of stem cells and severity of aGVHD. Finally, 3 variables increased the risk of progression during the entire posttransplantation period: prolonged duration of neutropenia prior to engraftment, advanced status of the underlying disease, and a short time interval (< 6 weeks) between start of AFT for the invasive aspergillosis and the allo-HSCT (not total duration of AFT but rather the interval from starting therapy and the day of transplantation). The use of voriconazole as up-front secondary prophylaxis showed a trend in reducing the risk of progression of IA (incidence of progression of IA in these 31 patients was 12% vs 22% in the other 98 patients; P = .15).
Risk of progression of IA after HSCT according to the first AFT given after transplantation as secondary prophylaxis
. | No. patients . | No. patients with progression of IA after allo-HSCT (%) . |
|---|---|---|
| First AFT given after transplantation* | ||
| Azoles (itraconazole/voriconazole) | 93 (50/43) | 24 (26) |
| AmB formulations (c-AmB/ABLC/L-AmB) | 58 (25/11/22) | 19 (33) |
| Caspofungin | 26 | 7 (27) |
| No AFT | 6 | 2 (33) |
| All patients at risk | 129† | 27 (21) |
. | No. patients . | No. patients with progression of IA after allo-HSCT (%) . |
|---|---|---|
| First AFT given after transplantation* | ||
| Azoles (itraconazole/voriconazole) | 93 (50/43) | 24 (26) |
| AmB formulations (c-AmB/ABLC/L-AmB) | 58 (25/11/22) | 19 (33) |
| Caspofungin | 26 | 7 (27) |
| No AFT | 6 | 2 (33) |
| All patients at risk | 129† | 27 (21) |
AmB indicates amphotericin B; and ABLC, AmB lipid complex.
Refers to the AFT used from the time of transplantation as first-line secondary prophylaxis or as second/third-line therapy after toxicity from prior therapy, without progression of the IA.
Forty-eight (37%) patients received more than 1 drug as first-line prophylaxis, either in combination or sequentially.
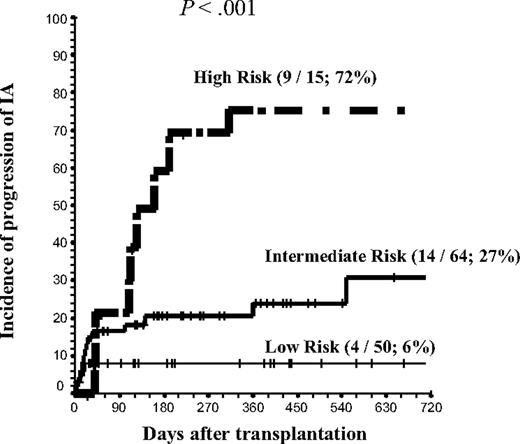
Using the major risk factors identified except intensity of the conditioning regimen (longer duration [> 20 days] of neutropenia after transplantation; advanced status of the underlying disease; time interval of < 6 weeks from start of systemic anti-Aspergillus therapy and the allo-HSCT; CMV disease; bone marrow or cord blood as source of stem cells; and grades II-IV aGVHD), a risk group categorization for progression of IA after allo-HSCT was calculated. The presence of 0 to 1, 2 to 3, or 4 to 6 risk factors identified low-risk, intermediate-risk, and high-risk group categories, respectively (Figure 3). When this risk model was applied separately to RIC and CONV groups, a clear prediction of the risk of progression of IA was found. Specifically, in RIC and CONV recipients, the incidence of progression in low-, intermediate-, and high-risk categories was 0%, 20%, and 60% in the RIC group and 13%, 26%, and 60% in the CONV group, respectively.
Since screening for serum GM during the posttransplantation period was performed in only 67 patients (52%), this variable was not included in the multivariate analysis. However, in the subgroup of patients screened, the presence of 2 or more indices of 0.8 or higher after transplantation was associated with progression of IA (11 [46%] of 24 vs 2 [4.7%] of 43).
Progression of IA before day 30 in recipients of a RIC or a CONV regimen. n = 13 patients. Tick marks indicate censored patients.
Progression of IA before day 30 in recipients of a RIC or a CONV regimen. n = 13 patients. Tick marks indicate censored patients.
NRM and OS
The major transplantation outcomes are shown in detail in Table 1. Of note, RIC recipients differed from CONV recipients in several characteristics and outcomes that were found to have an independent impact on the risk of progression of the IA after transplantation (detailed in Table 3). The most important differences were a higher proportion of PBSCs as a stem cell source, a shorter duration of profound neutropenia (and monocytopenia and lymphocytopenia; data not shown), and a lower incidence of grades II-IV aGVHD.
A detailed analysis of risk factors for NRM and OS is not given since it is not within the scope of this study. However, it is important to investigate whether progression of IA is an independent risk factor for both outcomes. The 2-year incidence of NRM was 30% (95% CI, 19%-39%), while the OS was 42% (95% CI, 30%-50%). In multivariate analyses, progression of IA after allo-HSCT (analyzed as a time-dependent variable) was found to be an independent risk factor for both outcomes (HR of 12.9, 95% CI of 4.2-39 for NRM and HR of 10.8, 95% CI 3.3-33 for OS). Other negative risk factors for both NRM and OS were advanced status of the underlying disease, age older than 50 years, grades III to IV aGVHD, and pretransplantation recipient CMV immunoglobulin G (IgG) seropositivity. Alternative HLA-mismatched donors had a negative impact on NRM only, and use of BM/CB as a stem cell source had a negative impact on OS only.
Landmark analysis of the 2-year cumulative incidence of progression of IA. Analysis included only patients alive and well on day 30 after BMT or CBT (A) or PBSCT (B) according to whether or not patients developed aGVHD grades II to IV. n=14 patients. Tick marks indicated censored patients.
Landmark analysis of the 2-year cumulative incidence of progression of IA. Analysis included only patients alive and well on day 30 after BMT or CBT (A) or PBSCT (B) according to whether or not patients developed aGVHD grades II to IV. n=14 patients. Tick marks indicated censored patients.
Progression of IA according to the risk group, defined by the number of risk factors per patient. Risk factors include (1) more than 20 days of neutropenia after transplantation; (2) advanced underlying disease at transplantation; (3) CMV disease; (4) time interval from start of AFT and transplantation less than 6 weeks; (5) bone marrow or cord blood as the source of stem cells; and (6) grades II to IV aGVHD that required high-dose steroids (prednisone or equivalent at ≥ 2 mg/kg for > 10 days) with or without ATG for treatment. Risk-group categories were low risk, 0 to 1 risk factors; intermediate risk, 2 to 3 risk factors; and high risk, 4 to 6 risk factors. Numbers in parentheses indicate number of patients who had progressive IA/number of patients in this risk group; 2-year cumulative incidence. Tick marks indicate censored patients.
Progression of IA according to the risk group, defined by the number of risk factors per patient. Risk factors include (1) more than 20 days of neutropenia after transplantation; (2) advanced underlying disease at transplantation; (3) CMV disease; (4) time interval from start of AFT and transplantation less than 6 weeks; (5) bone marrow or cord blood as the source of stem cells; and (6) grades II to IV aGVHD that required high-dose steroids (prednisone or equivalent at ≥ 2 mg/kg for > 10 days) with or without ATG for treatment. Risk-group categories were low risk, 0 to 1 risk factors; intermediate risk, 2 to 3 risk factors; and high risk, 4 to 6 risk factors. Numbers in parentheses indicate number of patients who had progressive IA/number of patients in this risk group; 2-year cumulative incidence. Tick marks indicate censored patients.
Discussion
Nearly all previous studies on the impact of a recent episode of IA on the outcome of allo-HSCT have included large proportions of patients classified as possible IA before transplantation (reviewed in detail by Martino et al18 ). This is partly due to the traditional very low rate of premortem diagnosis of IA with a high level of certainty before the more sensitive noninvasive diagnostic techniques were available.25-29 A review of 55 patients with possible, probable, or proven IA published before 1997 showed a very high rate of fatal reactivation with subsequent chemotherapy.18 However, when IA was in clinical and radiologic CR or PR, patients could safely receive further intensive chemotherapy or proceed to HSCT. On the other hand, when infection was not well controlled or when significant radiologic abnormalities persisted, these infections disseminated rapidly during subsequent myelosupression.
Publications that are more recent have mainly included small numbers of patients (< 10) with different IFIs who received further chemotherapy or autologous HSCT. However, these groups differ significantly from allo-HSCT patients with respect to outcome. In fact, only 2 studies have included more than 10 cases of well-documented IA prior to allo-HSCT (or autologous HSCT), with the largest group of patients followed only up to day 100 after transplantation.12,20
Thus, the current study has several strengths when compared with previously published studies, especially since it includes patients who received transplants recently, thus allowing an analysis of risk factors in an era of rapidly increasing improvements in supportive care. In addition, the relatively large number of patients and progressions of IA allowed us to perform reliable multivariate analyses and led to the identification of risk groups.
The incidence of posttransplantation progression of IA in the present study are somewhat lower than in previous EBMT reports and results from Seattle in allo-HSCT (35% and 29% at 1 year, respectively). Interestingly, univariate analysis from the Seattle data revealed that a pretransplantation AFT of less than 30 days, failure to achieve hematopoietic engraftment (which equals prolonged neutropenia), bone marrow transplantation–cord blood transplantation (BMT-CBT; vs PBSCT) and persistent radiographic abnormalities were associated with increased risks of progression of IA.20 The current study supports the conclusion that type of HSCT, longer time period from start of AFT for aspergillosis, and the allo-HSCT and response at the time of transplantation are important variables that predict outcome. The current data also add to our knowledge by demonstrating possible effects on outcome of the type of conditioning regimen used, severity of GVHD, and other post-HSCT complications (CMV disease). Unfortunately, due to the large number of antifungal regimens used after allo-HSCT, we were unable to analyze the impact of any given drug or combination therapy that may offer a better outcome. However, the observation for a trend in reducing the risk of progression of the IA with up-front secondary prophylaxis with voriconazole monotherapy is logical, since it is currently the only drug that has shown better efficacy than amphotericin B in the treatment of IA.22
From our study and previous data, several conclusions may be drawn. First, as suggested by our risk group categorization, a subset of patients with a recent history of IA exists with a low risk of recurrence after allogeneic transplantation. Second, RIC may reduce the risk of progression of IA after allo-HSCT. However, we realize that this finding should be interpreted with caution, since patients were selected for a RIC procedure on an individual basis in all different centers. Such a bias can lead to equivocal findings, especially in retrospective studies. Nevertheless, the lower risk of progression in RIC recipients is an encouraging observation when taking into account the larger proportion of patients with various high-risk features before transplantation in the RIC group. Finally, recent developments in the noninvasive diagnosis of IA may allow the earlier diagnosis of IA and/or the upgrading of a significant proportion of patients with possible IA into probable cases.27,28,30-34 Despite the heterogeneity of results on the usefulness and the interpretation of serum GM, numerous institutions are using this test in both the clinical and research settings. In a retrospective study, it is difficult to interpret the results of GM screening. However, our observations in the 67 patients who were regularly screened with serial serum GM after allo-HSCT are encouraging. Thus, 11 (46%) of 24 patients with 2 or more consecutive serum samples with a GM index of 0.8 or higher showed early progression of the IA (before day 30), while only 2 (4.7%) of 43 with negative serial GM had progression of the IA (ie, “falsely negative” GM screening).
Our study has several shortcomings that are due mainly to its retrospective nature. It is well known that such studies are subject to biases, which can sometimes be partially corrected by the large number of patients analyzed in retrospective international registry analyses. With this caveat in mind, it is reasonable to consider that RIC and CONV patients were heterogeneous regarding the exact types of conditioning regimens used and some important disease-related or conditioning-related variables, and, probably, patients' comorbidities other than prior IA.
In summary, our data reinforce the concept that a history of IA is not an absolute contraindication for allo-HSCT, especially if patients have a low risk profile, as identified in the current study. High-risk patients, on the contrary, may be ideal subjects for prospective studies aimed at reducing the high risk of progression of the IA. Our results may be useful in comparing the results of secondary antifungal prophylaxis by allowing more reliable risk group stratification for comparisons. Together with these variables, well-standardized laboratory methods to measure the activity of the IA after allo-HSCT (ie, daily GM sampling, blood polymerase chain reaction,35 or beta-D-glucan in serum36 ) may be useful in future strategies to reduce the risk of dying from IA after allo-HSCT in patients with a recent IA.
Appendix
Active IDWP members: Hamdi Akan, Turkey; Jan Apperly, United Kingdom; Mutlu Arat, Turkey; Stéphane Bretagne, France; Suparno Chakrabarti, United Kingdom; Catherine Cordonnier, France; Rafael de la Camara, Spain; Adrian W. Dekker, The Netherlands; Peter J. Donnelly, The Netherlands; Hermann Einsele, Germany; Dan Engelhard, Israel; Jörg Faber, Germany; Holger Hebart, Germany; Thomas K. Held, Germany; Ann Hunter, United Kingdom; Brian Jones, United Kingdom; Thomas Lehrnbecher, Germany; Hartmut Link, Germany; Per Ljungman, Sweden; Anna Locasciulli, Italy; Johan Maertens, Belgium; Rodrigo Martino, Spain; Shaun McCann, Ireland; Ellen Meijer, The Netherlands; Fritz Offner, Belgium; Jolanta Perz, Germany; Pierre Reusser, Switzerland; Patricia Ribaud, France; Malgorzta Rokicka, Poland; Montserrat Rovira, Spain; Peter J. Shaw, Australia; Marta Stanzani, Italy; Andre J. Ullman, Germany; Alvaro Urbano-Ispizua, Spain; Maria Teresa van Lint, Italy; Kate N. Ward, United Kingdom; and Dana Wolf, Israel.
Participants from the following centers entered patients in the current retrospective study (number of patients is in parentheses): T. Fukuda and K. A. Marr, Fred Hutchinson Cancer Research Center, Seattle, WA (16); R. Martino and R. Parody, Hematology Department, Sant Pau Hospital, Barcelona, Spain (15); J. Maertens and K. Theunissen, University Hospital Gasthuisberg, Leuven, Belgium (14); A. Ho and G. J. Mufti, King's College London School of Medicine, London, United Kingdom (14); N. Kröger and A. R. Zander, UKE Hamburg, Germany (11); D. Heim, University Hospital of Basel, Switzerland (8); M. Paluszewska, Medical University of Warsaw, Poland (6); D. Selleslag, AZ Sint Jan, Brugge, Belgium (5); K. Steinerova, Charles University Hospital, Pilsen, Czech Republic (4); P. Ljungman, Karolinska University Hospital, Stockholm, Sweden (4); S. Cesaro, Department of Pediatrics, Hospital of Padua, Italy (4); A. Nihtinen, University Central Hospital of Helsinki, Finland (4); C. Cordonnier, Henri Mondor Hospital, Creteil, France (3); L. Vazquez, Clinical Hospital of Salamanca, Spain (3); M. Lopez Duarte, Marqués de Valdecilla Hospital, Santander, Spain (3); J. Lopez, Ramón y Cajal Hospital, Madrid, Spain (3); R. Cabrera, Puerta de Hierro Hospital, Madrid, Spain (3); M. Rovira, Clinic Hospital of Barcelona, Spain (2); H. Einsele, Wuerzburg University Medical Center, Wuerzburg, Germany (2); S. Neuburger, Charité Campus Virchow Klinikum, Berlin, Germany (2); O. Cornely, University Hospital Cologne, Germany (1); N. Tabron, University Hospital of Leicester, United Kingdom (1); and H. J. Dornbusch, Division of Pediatric Hematology and Oncology, Medical University of Graz, Austria (1).
Prepublished online as Blood First Edition Paper, May 23, 2006; DOI 10.1182/blood-2006-03-008706.
Supported by in part by National Institutes of Health grants CA18029, CA78902, HL36444, and CA15704 (K.A.M. and T.F.); and by a grant of the Swiss National Research Roundation (3200B0-106105/1 to D.H.).
A complete list of the members of the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal