Abstract
T-cell subpopulations, defined by their expression of CD4, CD8, naive, and memory cell-surface markers, occupy distinct homeostatic compartments that are regulated primarily by cytokines. CD8+ memory T cells, as defined by CD44hi surface expression, are dependent on IL-15 as a positive regulator of their homeostatic maintenance. Manipulation of IL-15 signaling through gene aberration, overexpression, or receptor alterations has been shown to dramatically affect T-cell homeostasis, with overexpression leading to fatal leukemia. Here we show that TGF-β is the critical negative regulator of murine CD8+ memory T-cell homeostasis with direct opposition to the positive effects of IL-15. This negative regulation is mediated, at least in part, by the ability of TGF-β to modulate expression of the β-chain of the IL-15 receptor, thus establishing a central axis between these 2 cytokines for homeostatic control of CD8+ memory T-cell populations. These data establish TGF-β as a critical and dominant tumor-suppressor pathway opposing IL-15-mediated CD8+ T-cell expansion and potential malignant transformation.
Introduction
T cells can be divided into distinct, independently regulated subpopulations by cell-surface expression of CD4, CD8, naive, and memory cell-surface markers. Such subpopulations occupy distinct niches or compartments that maintain relatively constant numbers and ratios. Maintenance of such homeostatic balance is controlled by a combination of homeostatic proliferation and cell survival.1,2 Recently, several cytokines have been identified as important regulators in the maintenance of these T-cell compartments.1 Naive T cells have been shown to have increased survival in the presence of IL-4, IL-6, and IL-73-5 and are dependent on IL-7 for homeostatic proliferation.6,7 CD8+ memory T cells expressing the IL-2/IL-15 receptor β (IL-2/IL-15Rβ) chain (CD122) require IL-15 for survival and homeostatic proliferation.8-10 It has been suggested that for this cell subpopulation, IL-7 at high concentrations can substitute, or at least partially substitute, for IL-15.11 IL-2 and IL-4 can also regulate the CD8+ memory population but do not appear to be required for homeostasis.12-14 Cytokine regulation of CD4+ memory T cells is less clear. These T cells can be expanded in vivo by IL-7; however, they are also capable of expansion in common γ-chain receptor-deficient mice, suggesting that other mediators may act in memory CD4+ T-cell homeostasis.15
In contrast to the plethora of positively regulating cytokines affecting T-cell subpopulations, little is known of negative cytokine regulation that functions in T-cell homeostasis. It has been reported that IL-2 suppresses the division of memory T cells; however, this inhibitory effect appears to be mediated indirectly through CD25+CD4+ regulatory T cells.16 These regulatory cells are clearly responsible for some homeostatic T-cell regulation,17-19 but they cannot account in general for CD8+ T-cell regulation, which is not drastically influenced by the absence of IL-2 or CD4+ T cells.20,21 Alteration of the IL-15 signaling pathway, on the other hand, has a dramatic effect on CD8+ subpopulations, suggesting that other negatively regulating mechanisms must exist. Overproduction of IL-15 results in the gross expansion of the CD44hi, CD8+ memory T-cell population,22-24 and in one mouse model it resulted in T-cell leukemia.23
We generated a transgenic (Tg) mouse model (dominant-negative TGF-β II receptor transgenic [DNRII Tg] mice) in which T cells have a limited capacity for response to TGF-β and in which dysregulated growth of CD8+ memory T-cell populations has been observed.25,26 Using this model, which shares many characteristics with the IL-15 overproducers, the mechanism involved in dysfunctional homeostasis and leukemogenesis was investigated. In this study we show that TGF-β and IL-15 act as a central cytokine axis in maintaining homeostasis of CD8+ memory T-cell populations and that modulation of the IL-15Rβ chain by TGF-β may be involved in its IL-15-opposing effect. We conclude that TGF-β acts as a crucial and dominant tumor-suppressor cytokine that critically opposes IL-15-mediated CD8+ T-cell expansion and so counteracts homeostatic dysregulation, which can lead to malignant transformation.
Materials and methods
Mice
DNRII Tg mice were generated as previously described.25 Mutant strains of mice—CD4 knockout (KO), TAP-1 KO, and major histocompatibility class II (CII) KO—were obtained from The Jackson Laboratory (Bar Harbor, ME). IL-15 KO mice were a gift from Immunex (Seattle, WA). CD8β KO and 2C T-cell receptor (TCR) transgenic mice were a gift from Dr Dennis Loh (Washington University, St. Louis, MO). Male antigen HY (HY) TCR, Rag-2 KO mice were a gift from Dr Melanie Vacchio (NIH, Bethesda, MD). All mice were generated or backcrossed and maintained on a C57BL/6 background strain and were housed according to National Institutes of Health guidelines.
Cell populations
Splenocytes and lymph node cells were prepared as previously described.27 T-cell populations were purified from splenocytes by magnetic bead separation (Miltenyi Biotec, Auburn, CA) or by passage over an immunocolumn (Cedarlane, Burlington, NC). Purity of the T-cell populations was established by flow cytometric analysis and was always greater than 90%. Cells were maintained in complete culture medium—RPMI 1640 (Invitrogen, Carlsbad, CA) with 1 mM sodium pyruvate (Invitrogen), 100 mM nonessential amino acid (Invitrogen), 100 U/mL penicillin + 100 mg/mL streptomycin (Invitrogen), 50 mM 2-mercaptoethanol (Fisher Scientific, Pittsburgh, PA), 2 mM l-glutamine (Invitrogen), and 10% FBS (Hyclone, Logan, UT). Cells were incubated at 37°C with 5% CO2/95% air.
Flow cytometry
Biotin-conjugated, FITC-conjugated, and phycoerythrin-conjugated antibodies against mouse CD4/CD8 (Becton Dickinson, San Jose, CA), CD25/CD122/TNFRII/CD44 (PharMingen, San Diego, CA), anti-IL-15Rα (R&D Systems, Minneapolis, MN), and anti-HY TCR (T370) were used to stain cells from lymph node and spleen. Biotin-conjugated (1 μg) antibodies were incubated in a total volume of 20 μL for 20 minutes on ice, washed once in flow cytometry media (Hanks balanced salt solution [Invitrogen] plus 1% BSA [Sigma, St Louis, MO] plus 1% azide [wt/vol; Sigma]). An FcR-specific antibody, 2.4G2, was used to block FcR binding. CFSE-labeled and antibody-stained cells were analyzed as previously reported.27 Data are presented on log scale as either histogram or dot plot.
Adoptive transfer experiments
PBS-washed lymphocytes or purified T cells derived from lymph node or spleen were resuspended at 10 × 106 cell/mL in PBS and were incubated with 1 μM CFSE (Molecular Probes, Eugene, OR) for 15 minutes at 37°C. Labeled cells were centrifuged at 1000g for 10 minutes, resuspended in PBS for 30 minutes at 37°C, washed once in PBS, and resuspended at 50 × 106 cell/mL in PBS. CFSE-labeled cells (5 × 106) were injected into recipient mice through the tail vein using a 30-gauge needle. Lymph node cells and splenocytes were harvested 7 to 14 days later for flow cytometric analysis. In vivo IL-7 delivery was performed using Alzet Mini-Osmotic Pumps (Durect, Cupertino, CA) that delivered 5 μg/d murine recombinant IL-7 (PeproTech, Rocky Hill, NJ) for 7 to 14 days. Pumps were implanted subcutaneously between the scapulae.
In vitro IL-15 culture
One million to 2 million CD8 Cellect Immunocolumn (Cedarlane, Hornby, ON, Canada)-purified CD8+ lymph node cells or splenocytes were placed in a 24-well plate (Costar, Corning, NY) in 1-mL complete culture medium with PBS, 100 ng/mL murine IL-15 (PeproTech), 40 ng/mL murine TGF-β1 (PeproTech), or a combination of IL-15 and TGF-β1. Optimal concentrations for IL-15 proliferation and inhibition by TGF-β were determined by a tritiated thymidine incorporation assay (data not shown). Cells were incubated various times at 37°C and 5% CO2, collected, centrifuged, and resuspended in FACS media for antibody staining.
For proliferation, CFSE-labeled (0.2 μM) purified CD8+ lymph node cells or splenocytes were placed in a 24-well plate (Costar) in 1 mL complete culture medium with PBS, 100 ng/mL murine IL-15 (PeproTech), 40 ng/mL murine TGF-β1 (PeproTech), or a combination of IL-15 and TGF-β1. Cells were incubated various times at 37°C and 5% CO2, collected, centrifuged, and resuspended in FACS media for antibody staining.
Results
DNRII Tg mice are characterized by dysregulated CD8+ memory T-cell growth
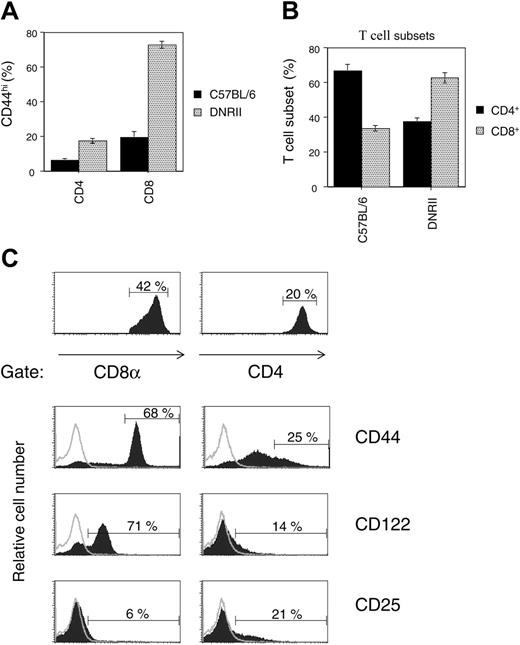
The DNRII Tg mouse expresses a T-cell-specific, dominant-negative transforming growth factor receptor-β2 transgene that results in a preferential increase in the percentage of T cells expressing the CD44hi memory cell marker on their cell surfaces (Figure 1A). As observed, T cells in DNRII Tg mice have increased CD44hi expression when compared with C57BL/6 mice, in CD4+ (6.3% vs 17%) and CD8+ (18% vs 75%) T-cell subsets; however, only the CD44hiCD8+ T-cell subset undergoes expansion over time, resulting in an increased percentage of total CD8+ T cells (Figure 1B) and an absolute increase in cell number.25 This expansion correlates with the coexpression of the β-chain of the IL-2/IL-15R (CD122) without increased surface expression of IL-2R-α (CD25) (Figure 1C), suggesting that the CD8+ memory T-cell expansion is IL-15 driven. No differences in IL-2R-α or -γ expression levels were observed between CD4+ and CD8+ T cells (Figure 1C; data not shown).
DNRII Tg CD8+ T-cell expansion requires antigen and MHC
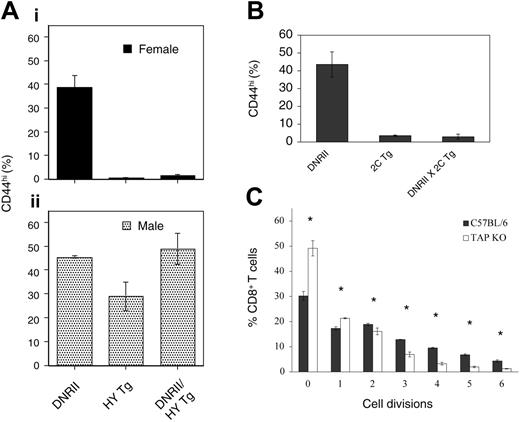
CD8+CD44hi T cells predominantly belong to the memory T-cell subset.28 One exception has been naive CD8+ T cells that upregulate CD44 surface expression when undergoing homeostatic proliferation in a lymphopenic host.29-31 To distinguish homeostatic expansion of naive T cells from antigen-driven conversion to memory phenotype with uncontrolled expansion of the CD8+ DNRII T cells, female mice bearing a Tg-αβ TCR specific for the HY male antigen, deficient in the Rag2 gene, and expressing the DNRII transgene were analyzed for CD44hi expression and compared with littermates expressing each transgene alone (Figure 2A, top panel). T cells from HY transgenic mice do not undergo homeostatic expansion32,33 ; if expansion of T cells from DNRII Tg mice require antigen, then no expansion should be observed. These studies showed that the CD44hi phenotype associated with the DNRII transgene does not occur in the presence of an αβ TCR of defined HY antigenic specificity and in the absence of the specific ligand for that TCR, suggesting that antigenic stimulation is required for the conversion of naive DNRII CD8+ T cells to a memory phenotype and the development of the expanded CD44hiCD8+ T-cell populations constituting the phenotype of these mice. This naive phenotype can be converted to a memory/CD44hi expression in the presence of HY antigen, as is evident in male, HY Tg, RAG-2 KO mice (Figure 2A, bottom panel). Male HY Tg, RAG-2 KO mice expressing the DNRII transgene have an increased percentage of CD44hi, TCR-αβ, CD8+ T cells and increased lymphocyte cellularity compared with HY Tg, RAG-2 KO female littermates (Figure 2A, bottom panel; data not shown).
CD44hiCD8+T lymphocytes preferentially expand in DNRII Tg mice. (A) Lymphocytes from 6-week-old DNRII Tg mice or nontransgenic littermates (C57BL/6) were analyzed for CD44hi cell-surface expression (MFI, greater than 100) on CD4+ or CD8+ T-cell subsets. Data are represented as the average percentage of CD44hi cells in each T-cell subset, with error bars representing the SD in each group. (B) Lymphocytes from the same 6-week-old DNRII Tg mice or nontransgenic littermates (C57BL/6) were analyzed for the percentages of CD4+ or CD8+ T-cell subsets. Data are represented as the average percentage of each T-cell subset among the total T-cell population, with error bars representing the SD in each group. (C) Lymphocytes from 6-week-old DNRII Tg mice were gated into CD8+ T-cell subsets (left) or CD4+ T-cell subsets (right). Data in the top 2 panels represent the percentage of each T-cell subset with respect to total T cells in the sample. The bottom 6 panels represent flow cytometric analysis of surface receptor expression in each of the 2 gated T-cell subsets (filled histograms). Data represent the percentages of indicated surface receptors within each gated T-cell subset and are typical of these strains of mice at 6 weeks of age. Open histograms indicate nonspecific control staining. (A-B) Average of 5 or more mice per group; experiments repeated 5 times.
CD44hiCD8+T lymphocytes preferentially expand in DNRII Tg mice. (A) Lymphocytes from 6-week-old DNRII Tg mice or nontransgenic littermates (C57BL/6) were analyzed for CD44hi cell-surface expression (MFI, greater than 100) on CD4+ or CD8+ T-cell subsets. Data are represented as the average percentage of CD44hi cells in each T-cell subset, with error bars representing the SD in each group. (B) Lymphocytes from the same 6-week-old DNRII Tg mice or nontransgenic littermates (C57BL/6) were analyzed for the percentages of CD4+ or CD8+ T-cell subsets. Data are represented as the average percentage of each T-cell subset among the total T-cell population, with error bars representing the SD in each group. (C) Lymphocytes from 6-week-old DNRII Tg mice were gated into CD8+ T-cell subsets (left) or CD4+ T-cell subsets (right). Data in the top 2 panels represent the percentage of each T-cell subset with respect to total T cells in the sample. The bottom 6 panels represent flow cytometric analysis of surface receptor expression in each of the 2 gated T-cell subsets (filled histograms). Data represent the percentages of indicated surface receptors within each gated T-cell subset and are typical of these strains of mice at 6 weeks of age. Open histograms indicate nonspecific control staining. (A-B) Average of 5 or more mice per group; experiments repeated 5 times.
The preceding experiments show that antigen stimulation is sufficient to produce the phenotype of CD8+ T-cell expansion observed in DNRII Tg mice but do not address whether it is necessary because, as noted, homeostatic proliferation does not occur. Consequently, to further examine the role of antigen-specific recognition, a second TCR Tg mouse model was investigated. The 2C TCR transgenic mouse model contains a transgenic TCR, which, when expressed on CD8 T cells, allows recognition of the alloantigen MHC class I Ld molecule. This TCR/MHC alloresponse has been shown to involve a specific self-peptide bound to the Ld molecule that does not cross-react with environmental antigens.34 Similar to the results obtained with the HY TCR transgenic mice model, the conversion of naive DNRII CD8+ T cells to a memory phenotype and the development of the expanded CD44hiCD8+ T cells did not occur when coexpressed with the 2C TCR transgene (Figure 2B), supporting the hypothesis that peptide-MHC interactions are required for the development of CD8+ T cells to a memory phenotype found in DNRII Tg mice.
Antigenic requirements for CD8+ T-cell expansion in DNRII Tg mice. (A) Lymphocytes from 8- to 10-week-old female (i) and male (ii) DNRII Tg, HY TCR Tg, or DNRII, HY TCR double-transgenic mice were analyzed for CD44hi (MFI, greater than 100) cell-surface expression on the CD8+ T-cell population. Data are represented as the average percentage of CD44hi cells in each T-cell subset; error bars represent the SD in each group (each group included 3 or more mice). (B) Lymphocytes from 8- to 10-week-old female DNRII Tg, 2C TCR Tg, or DNRII, 2C TCR double-transgenic mice were analyzed for CD44hi (MFI, greater than 100) cell-surface expression on the CD8+ T-cell population. Data are represented as the average percentage of CD44hi cells in each T-cell subset; error bars represent the SD in each group (each group included 3 or more mice). (C) Purified, CFSE-labeled DNRII Tg T cells from 10-week-old homozygous Tg mice were injected into TAP-1 KO or age-matched C57BL/6 hosts. Hosts were killed, and lymphocytes from lymph nodes were pooled and analyzed for CFSE+ T cells 1 week after injection. Data are represented as the average percentage of CFSE+, CD8+ T cells in each cell division group. Error bars represent the SD in each group (n = 3), with asterisks denoting significant (P < .05) differences between C57BL/6 and Tap KO hosts. The experiment was performed 3 times with similar results (total n = 10/group).
Antigenic requirements for CD8+ T-cell expansion in DNRII Tg mice. (A) Lymphocytes from 8- to 10-week-old female (i) and male (ii) DNRII Tg, HY TCR Tg, or DNRII, HY TCR double-transgenic mice were analyzed for CD44hi (MFI, greater than 100) cell-surface expression on the CD8+ T-cell population. Data are represented as the average percentage of CD44hi cells in each T-cell subset; error bars represent the SD in each group (each group included 3 or more mice). (B) Lymphocytes from 8- to 10-week-old female DNRII Tg, 2C TCR Tg, or DNRII, 2C TCR double-transgenic mice were analyzed for CD44hi (MFI, greater than 100) cell-surface expression on the CD8+ T-cell population. Data are represented as the average percentage of CD44hi cells in each T-cell subset; error bars represent the SD in each group (each group included 3 or more mice). (C) Purified, CFSE-labeled DNRII Tg T cells from 10-week-old homozygous Tg mice were injected into TAP-1 KO or age-matched C57BL/6 hosts. Hosts were killed, and lymphocytes from lymph nodes were pooled and analyzed for CFSE+ T cells 1 week after injection. Data are represented as the average percentage of CFSE+, CD8+ T cells in each cell division group. Error bars represent the SD in each group (n = 3), with asterisks denoting significant (P < .05) differences between C57BL/6 and Tap KO hosts. The experiment was performed 3 times with similar results (total n = 10/group).
To further investigate whether peptide-MHC complexes are involved in the observed CD8+ memory T-cell expansion in DNRII Tg animals, adoptive transfer experiments were used. CFSE-labeled DNRII T cells were transferred into TAP-1 KO hosts (Figure 2C), which lack a transport protein needed for endogenous peptide to be loaded into the MHC class I molecule, thus allowing study of the effect of peptide alone on DNRII CD8+ T-cell proliferation. DNRII CD8+ T cells transferred to unmanipulated (control) C57BL/6 hosts (Figure 2C) divided up to 6 times in a 1-week period, which is consistent with these cells being of memory phenotype.35-37 However, when CD8+ DNRII T cells were transferred to a TAP KO host, significantly fewer CFSE+/CD8+ T cells divided after transfer (Figure 2C). These data are consistent with a need for persistent peptide-MHC class I interactions for sustained proliferation of the DNRII memory CD8+ T cells. Injected CD4+ T cells were used as a control for cell recovery and were maintained at similar levels in C57BL/6 and TAP KO hosts (data not shown).
CD4+ T-cell subpopulations are not required for DNRII CD8+ T-cell proliferation
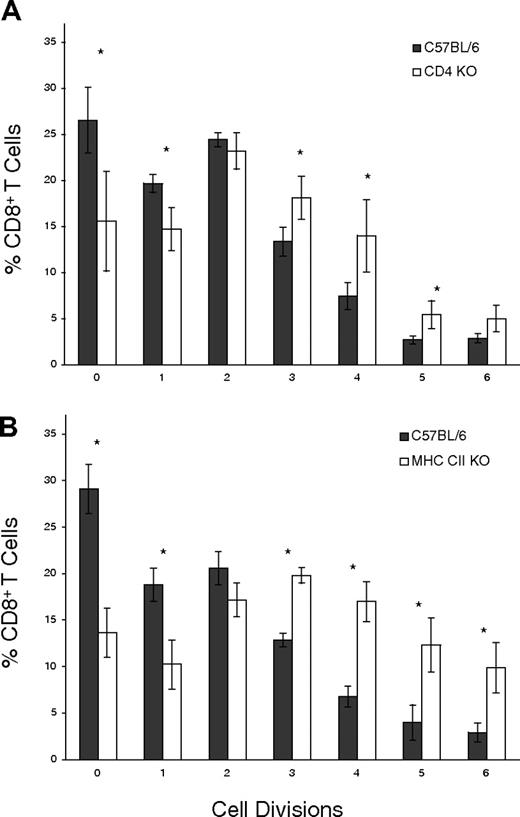
The results shown define an antigen-driven, memory CD8+ T-cell expansion pathway profoundly influenced by TGF-β signaling. To further understand the forces affecting such homeostasis, it is important to experimentally define the contributions of different T-cell subpopulations that have been described to influence T-cell population dynamics.38-40 The role of CD4+ T-cell subsets was addressed by adoptively transferring CFSE-labeled DNRII CD8+ T cells into either CD4 KO or MHC CII KO hosts. When CFSE-labeled, DNRII CD8+ T cells were examined after 10 days, DNRII CD8+ T-cell proliferation was found not to have been negatively affected by CD4 KO (Figure 3A) or MHC CII KO (Figure 3B) hosts, in contrast to the C57BL/6 host. Rather, significantly more cells underwent one or more cell divisions (Figure 3A-B), indicating that CD4+ T-cell subsets are not required for the expansion of CD8+ memory T cells when TGF-β signaling has been disrupted and, in fact, may be involved in preventing more rapid CD8+ memory T-cell expansion.
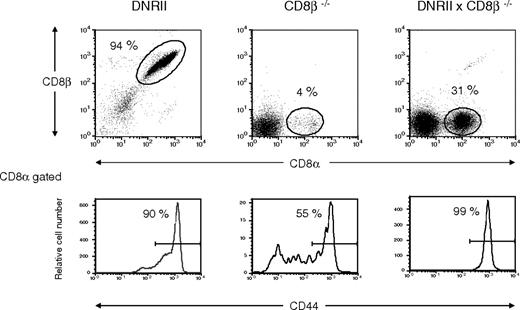
No abnormalities in CD4+ T-cell homeostasis were initially observed in DNRII Tg mice. To further address CD4+ T-cell homeostasis in these mice, DNRII, CD8β chain KO mice were studied. CD8β KO mice have defective thymocyte maturation that prevents CD8+ T cells from developing and migrating to the periphery.41 CD4+ T cells from DNRII, CD8β KO mice were not increased over control mice, suggesting that a regulatory CD8αβ+ T cell was not responsible for the lack of CD4+ T-cell expansion in the DNRII Tg mouse (data not shown); this observation further supports a specific role for TGF-β in CD8+ compared with CD4+ T-cell homeostasis. Interestingly, CD8+ T cells from DNRII, CD8β KO mice, which express TCR-αβ and CD8αα receptors, respond to the presence of a dominant-negative TGF-β type II receptor in much the same manner as T cells expressing TCR-αβ and CD8αβ receptors with an increased percentage (Figure 4) and number (data not shown) of CD44hiCD8+ T cells. This suggests that CD8αα and CD8αβ memory T-cell expansion is TGF-β regulated.
Interdependence of CD4+ and CD8+ T-cell subsets. (A) Purified, CFSE-labeled, DNRII Tg T cells from 8-week-old mice were injected into CD4 KO hosts (□) or age-matched C57BL/6 hosts (▪). Hosts were killed, and lymphocytes from lymph nodes were pooled and analyzed for CFSE+ T cells 10 days after injection. Data are represented as the average percentage of CFSE+, CD8+ T cells in each cell division group. Error bars represent the SD in each group (n = 5), and asterisks denote significant (P < .05) differences between C57BL/6 and CD4 KO hosts. (B) Purified, CFSE-labeled DNRII Tg T cells from 8-week-old mice were injected into MHC class II KO hosts (□) or age-matched C57BL/6 hosts (▪). Hosts were killed, and lymphocytes from lymph nodes were pooled and analyzed for CFSE+ T cells 10 days after injection. Data are represented as the average percentage of CFSE+, CD8+ T cells in each cell division group. Error bars represent the SD in each group (n = 5), and asterisks denote significant (P < .05) differences between C57BL/6 and MHC class II KO hosts.
Interdependence of CD4+ and CD8+ T-cell subsets. (A) Purified, CFSE-labeled, DNRII Tg T cells from 8-week-old mice were injected into CD4 KO hosts (□) or age-matched C57BL/6 hosts (▪). Hosts were killed, and lymphocytes from lymph nodes were pooled and analyzed for CFSE+ T cells 10 days after injection. Data are represented as the average percentage of CFSE+, CD8+ T cells in each cell division group. Error bars represent the SD in each group (n = 5), and asterisks denote significant (P < .05) differences between C57BL/6 and CD4 KO hosts. (B) Purified, CFSE-labeled DNRII Tg T cells from 8-week-old mice were injected into MHC class II KO hosts (□) or age-matched C57BL/6 hosts (▪). Hosts were killed, and lymphocytes from lymph nodes were pooled and analyzed for CFSE+ T cells 10 days after injection. Data are represented as the average percentage of CFSE+, CD8+ T cells in each cell division group. Error bars represent the SD in each group (n = 5), and asterisks denote significant (P < .05) differences between C57BL/6 and MHC class II KO hosts.
IL-15 is responsible for most DNRII CD8+ T-cell division
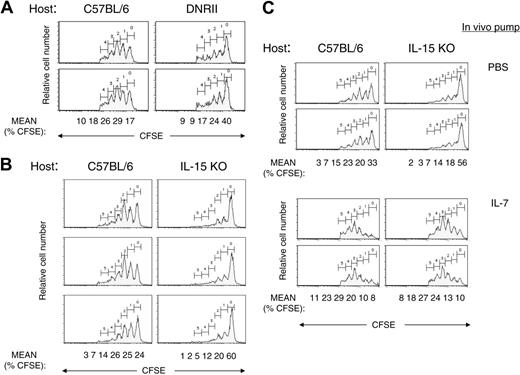
To study the general influence of cytokines in the in vivo environment on DNRII CD8+ T-cell expansion, an adoptive transfer of CFSE-labeled DNRII CD8+ T cells into DNRII Tg hosts was performed. DNRII CD8+ T cells, when transferred back into DNRII Tg hosts, did not proliferate as well as in C57BL/6 hosts (Figure 5A). These data are consistent with a hypothesis that an environmental cytokine is important in driving CD8+ T-cell proliferation and that this cytokine is limiting in DNRII Tg mice, possibly because of consumption by other CD8+ T cells. One factor, consistent with all data, would be the non-T-cell-derived cytokine IL-15, which is produced by mostly nonlymphoid tissues and activated monocytes/macrophages and is expressed at low serum levels in normal mice.42 Additionally, mice deficient in IL-15 have a striking decrease in the number of CD8+CD44hi T cells, whereas Tg mice with IL-15 overexpression have an increase in CD8+CD44hi T cells.10,22,43
To investigate the in vivo role of IL-15 on DNRII CD8+ T-cell expansion, CFSE-labeled DNRII T cells were transferred into IL-15 KO hosts. Transferred DNRII CD8+ T cells consistently proliferated less well (P = .01) in IL-15 KO hosts (mean ± SD, 39.2% ± 0.3%) compared with C57BL/6 control hosts (74.8% ± 6%) (Figure 5B), confirming the hypothesis that IL-15 is a major cytokine involved in the expansion of DNRII memory CD8+ T cells. The limited cell division that was observed in IL-15 KO hosts was consistent among experiments and might have reflected a residual effect of IL-15 from the donor.44 Alternatively, this effect might have indicated the presence of another factor that can drive CD8+ memory T-cell expansion. One possible cytokine known to affect CD8+ memory T-cell proliferation is IL-7.6 To investigate the role of IL-7 in the IL-15 KO host, antibody known to block IL-7 function in vivo was added to the IL-15 KO hosts before and after transfer of the DNRII CD8+ T cells at a dose known to inhibit T-cell function.45,46 No additional effect on CD8+ T-cell proliferation was observed (data not shown). Therefore, physiologic levels of IL-7 appear to play a minor role, if any, in the TGF-β control of CD8+ T-cell memory homeostasis. However, exogenous IL-7 added to the IL-15 KO hosts after the adoptive transfer of DNRII T cells restored full proliferative capacity to the transferred T cells (Figure 5C), suggesting that exogenous IL-7, at nonphysiologic levels, is sufficient to replace IL-15 in memory DNRII CD8+ T-cell expansion, as recently reported for other CD8+ memory T-cell populations.11
TGF-β regulates IL-2/IL-15Rβ levels
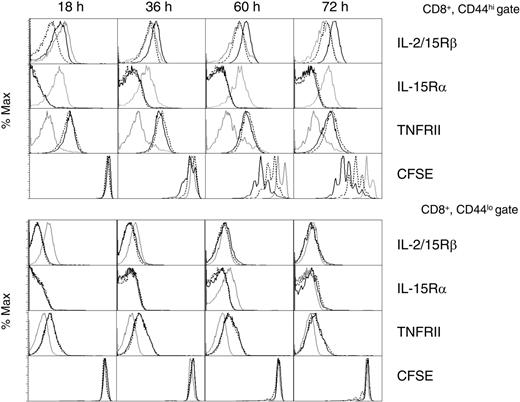
Because the primary phenotype of the DNRII transgenic CD8+ T cells was an increase in CD44hiCD122hi T cells, the role of TGF-β regulation of IL-2/IL-15Rβ was investigated in nontransgenic CD8+ T cells. Purified T cells were incubated with media, IL-15, or IL-15 + TGF-β for various times; this was followed by flow cytometric analysis of IL-2/IL-15Rβ, IL-15Rα, and TNF receptor II (TNFRII) on CD8+CD44hi T cells (Figure 6, top panels) and CD8+CD44lo T cells (Figure 6, bottom panels). IL-15 greatly enhanced cell-surface IL-2/IL-15Rβ levels beginning at 36 hours (MFI, 53 vs 32 for media control) on CD44hi, but not CD44lo, CD8+ T cells (Figure 6). The addition of TGF-β completely prevented this upregulation at all time points and appeared to suppress IL-2/IL-15Rβ levels in the CD44hiCD8+ T-cell population at the earliest time point (18 hours). TGF-β alone did not significantly downregulate IL-2/IL-15Rβ at any time point (data not shown), suggesting that TGF-β synergizes with IL-15 for the downregulation observed at the 18-hour time point. IL-15 also upregulated TNFRII surface expression, but, unlike IL-2/IL-15Rβ, this receptor was not inhibited by TGF-β, confirming that TGF-β did not function by the downregulation of all cytokine receptors. Similarly, IL-15 stimulation did not result in the upregulation of all cytokine receptors, as evident with the downregulation of IL-15Rα.47 This IL-15-induced downregulation of IL-15Rα expression may function by allowing IL-2/IL-15Rβ to act as the dominant receptor-signaling molecule. Cell viability studies showed that TGF-β did not function by increased cell death (data not shown). In addition, in vitro IL-15-induced proliferation of these purified DNRII CD8+CD44hi T cells, as measured by CFSE (Figure 6), demonstrated that upregulated IL-2/IL-15Rβ is functional and is negatively affected in the presence of TGF-β (cells with more than 2 divisions, 70% [IL-15] vs 14% [IL-15+TGF-β] at 60 hours and 82% vs 31% at 72 hours). Little cell division was detected in the CD8+CD44lo T-cell population in which IL-2/IL-15Rβ was not upregulated in the presence of IL-15 (Figure 6). Together these data offer a potential mechanism for the increased proliferation of CD8+ memory T cells observed in DNRII transgenic mice. In normal CD44hiCD8+ T cells, TGF-β has been shown to regulate the levels of IL-2/IL-15Rβ, thus controlling the ability of IL-15 to affect these cells. In the absence of TGF-β, IL-2/IL-15Rβ increases over time in response to IL-15, allowing for an enhanced response to this cytokine.
Flow cytometric analysis of CD8+ T cells from CD8β KO × DNRII Tg mice. Lymphocytes from a representative CD8β KO × DNRII Tg mouse (right panel), a CD8β KO mouse (center panel), and a DNRII Tg mouse (left panel) were analyzed for CD8α and CD8β chains (top panels) or CD44 surface expression on the CD8α-gated lymphocytes (bottom panels). Percentages of gated CD8α+ cells (top panels) are denoted by the numbers adjacent to the indicated oval gates and are represented as percentages of total lymphocyte numbers. Percentages of CD44hi cells in the CD8α population (bottom panels) are indicated to the left of the CD44hi gates. Data represent typical (n > 10) flow cytometric analysis of CD8α+ cells from these strains of mice. All mice studied were older than 16 weeks of age.
Flow cytometric analysis of CD8+ T cells from CD8β KO × DNRII Tg mice. Lymphocytes from a representative CD8β KO × DNRII Tg mouse (right panel), a CD8β KO mouse (center panel), and a DNRII Tg mouse (left panel) were analyzed for CD8α and CD8β chains (top panels) or CD44 surface expression on the CD8α-gated lymphocytes (bottom panels). Percentages of gated CD8α+ cells (top panels) are denoted by the numbers adjacent to the indicated oval gates and are represented as percentages of total lymphocyte numbers. Percentages of CD44hi cells in the CD8α population (bottom panels) are indicated to the left of the CD44hi gates. Data represent typical (n > 10) flow cytometric analysis of CD8α+ cells from these strains of mice. All mice studied were older than 16 weeks of age.
Discussion
Regulation of T-cell homeostasis is dominated by various cytokines exerting mostly proliferative and antiapoptotic influences on the different T-cell subpopulations. Many of these cytokines, if left unopposed, have the potential to lead to aberrant expansion of T-cell subsets and possibly malignant transformation. In contrast, TGF-β is a well-described tumor suppressor that has the opposite effect on T cells. It is antiproliferative and proapoptotic, which makes it an ideal candidate for opposing cytokines involved in T-cell expansion.
In our study, TGF-β signaling is inhibited by dominant-negative TGF-β II receptors, resulting in a distinct dichotomy between CD4+ and CD8+ T-cell homeostasis. This dichotomy results primarily from the TGF-β/IL-15 axis of cytokine regulation for CD8+ memory T cells, as demonstrated by a significant reduction of DNRII Tg CD8+ T-cell division in the absence of IL-15. IL-15 is a known positive homeostatic regulator that drives the expansion of antigen-activated (memory) CD8+ T cells, and TGF-β is a known immunosuppressive molecule that, in these experiments, opposed the IL-15-induced expansion of CD44hiCD122+CD8+ memory T cells. Consistent with this model, we show that T cells from TCR transgenic mice, which have not encountered cognate antigen, do not expand when TGF-β signaling is disrupted, whereas T cells from mice with a normal TCR repertoire, and thus the potential to respond to environmental antigens, do expand. The expansion of the CD8αα+ T-cell population may appear to contradict this model, but a recent study has shown that the expression of CD8αα homodimers is a marker for memory T cells,48 supporting the idea that the expanded T cells have previously encountered antigen. Thus, our data suggest that IL-15 levels in a mouse that has not recently encountered antigen are not limiting with respect to CD8+ memory T-cell expansion potential but instead are opposed by TGF-β cytokine levels.
Factor-dependent expansion of CD8+ DNRII Tg T cells. (A) Purified, CFSE-labeled DNRII Tg T cells from 8- to 10-week-old mice were injected into DNRII Tg (right panels) or age-matched C57BL/6 host (left panels). Hosts were killed, and lymphocytes from lymph nodes were pooled and analyzed for CFSE+ T cells 2 weeks after injection. Data shown are from 2 representative mice. Mean of the percentage of CD8+ T cells in each peak is shown below the last row of profiles. (B) Purified, CFSE-labeled DNRII Tg T cells from 8- to 10-week-old mice were injected into IL-15 KO (right panels) or age-matched C57BL/6 host (left panels). Hosts were killed, and lymphocytes from lymph nodes were pooled and analyzed for CFSE+ T cells 2 weeks after injection. Lymphocytes from lymph nodes were analyzed by flow cytometric analysis for CFSE staining. Data shown are for CD8+ T cells from 3 representative mice. Mean of the percentage of CD8+ T cells in each peak is shown below the last row of profiles. (C) Purified, CFSE-labeled DNRII Tg T cells from 8- to 10-week-old mice were injected into IL-15 KO (right panels) or age-matched C57BL/6 hosts (left panels) with subcutaneously implanted mini-osmotic pumps containing either PBS (top panels) or IL-7 (bottom panels). Hosts were killed, and lymphocytes from lymph nodes were pooled and analyzed for CFSE+ T cells 2 weeks after injection. Data shown are for CD8+ T cells from 2 representative mice per group. Mean of the percentage of CD8+ T cells in each peak is shown below the last row of profiles. All experiments were repeated at least 3 times with similar results.
Factor-dependent expansion of CD8+ DNRII Tg T cells. (A) Purified, CFSE-labeled DNRII Tg T cells from 8- to 10-week-old mice were injected into DNRII Tg (right panels) or age-matched C57BL/6 host (left panels). Hosts were killed, and lymphocytes from lymph nodes were pooled and analyzed for CFSE+ T cells 2 weeks after injection. Data shown are from 2 representative mice. Mean of the percentage of CD8+ T cells in each peak is shown below the last row of profiles. (B) Purified, CFSE-labeled DNRII Tg T cells from 8- to 10-week-old mice were injected into IL-15 KO (right panels) or age-matched C57BL/6 host (left panels). Hosts were killed, and lymphocytes from lymph nodes were pooled and analyzed for CFSE+ T cells 2 weeks after injection. Lymphocytes from lymph nodes were analyzed by flow cytometric analysis for CFSE staining. Data shown are for CD8+ T cells from 3 representative mice. Mean of the percentage of CD8+ T cells in each peak is shown below the last row of profiles. (C) Purified, CFSE-labeled DNRII Tg T cells from 8- to 10-week-old mice were injected into IL-15 KO (right panels) or age-matched C57BL/6 hosts (left panels) with subcutaneously implanted mini-osmotic pumps containing either PBS (top panels) or IL-7 (bottom panels). Hosts were killed, and lymphocytes from lymph nodes were pooled and analyzed for CFSE+ T cells 2 weeks after injection. Data shown are for CD8+ T cells from 2 representative mice per group. Mean of the percentage of CD8+ T cells in each peak is shown below the last row of profiles. All experiments were repeated at least 3 times with similar results.
TGF-β regulation of IL-2/IL-15Rβ expression on CD8+ memory T cells. Purified CD8+ T cells from 8- to 10-week-old C57BL/6 mice were incubated in complete mouse media (gray lines), +IL-15 (black lines), or +IL-15 plus TGF-β (dotted lines) for various times. Cell-surface expression of IL-2/IL-15Rβ (first row), IL-15Rα (second row), and TNFRII (third row) were determined by flow cytometric analysis on the CD8+, CD44hi T-cell population (top panels) and the CD8+CD44lo T-cell population (bottom panels). CFSE-labeled CD8+ T cells were included in each experiment to measure cell division (bottom row). Experiments were repeated 4 times with similar results.
TGF-β regulation of IL-2/IL-15Rβ expression on CD8+ memory T cells. Purified CD8+ T cells from 8- to 10-week-old C57BL/6 mice were incubated in complete mouse media (gray lines), +IL-15 (black lines), or +IL-15 plus TGF-β (dotted lines) for various times. Cell-surface expression of IL-2/IL-15Rβ (first row), IL-15Rα (second row), and TNFRII (third row) were determined by flow cytometric analysis on the CD8+, CD44hi T-cell population (top panels) and the CD8+CD44lo T-cell population (bottom panels). CFSE-labeled CD8+ T cells were included in each experiment to measure cell division (bottom row). Experiments were repeated 4 times with similar results.
A corollary to this hypothesis would be that CD8+ memory T-cell expansion in the presence of a TGF-β signal would normally occur as a result of increased IL-15 levels and that TGF-β levels are primarily responsible for maintaining homeostasis. This hypothesis is consistent with published results demonstrating CD8+ T-cell expansion in response to infectious agents known to stimulate interferon-induced IL-15 production.8,49,50 Similarly, increased IL-15 production is known to increase IFN-γ production, leading to the downregulation of TGF-β signaling through the SMAD-7 pathway and thus to a feedback loop that enhances the effect of the IL-15 production.51,52 In this setting, IL-15 alone could act as a homeostatic cytokine, much as IL-7 appears to do for CD4+-naive T cells.7
Although our data definitively demonstrate that IL-15 is the major cytokine responsible for CD8+ memory T-cell expansion when TGF-β signaling is limiting, they do not address the role of TGF-β in the initial conversion of CD8+ T cells to memory phenotype. In addition, other cytokines, such as IL-7 and IL-2, have the potential to influence the TGF-β/IL-15 axis of regulation. This is demonstrated in Figure 5C. Excess IL-7 can replace IL-15-induced proliferation of DNRII CD8+ T cells despite the inability to do so under normal physiologic conditions (Figure 5B), suggesting that increased IL-7 levels, such as those produced in a lymphopenic host, may replace IL-15 in opposing the negative influence of TGF-β signaling. Similarly, the opposing action of IL-15 on CD8+ T memory cells is reversed by IL-216,37 through a CD4+CD25+ regulatory pathway, suggesting that alternative negative homeostatic pathways may exist. This possibility is supported by a study demonstrating that DNRII T cells are susceptible to negative regulation of CD4+CD25+ regulatory T cells53 and by our current data showing an increase in CD8 T-cell proliferation in the absence of CD4+ T cells (Figure 3A-B). However, as shown in these experiments with the phenotype of the DNRII Tg mouse, this pathway of negative regulation does not have a major influence when TGF-β signaling is limited, demonstrating that the TGF-β/IL-15 axis of regulation is dominant under normal physiologic conditions.
The negative effects of TGF-β on hematopoiesis-derived cells have been extensively studied and involve numerous mechanisms of action, depending on cell type and environment. Growth arrest in many TGF-β-sensitive cells occurs through G1-phase growth arrest through TGF-β-mediated upregulation of cyclin-dependent kinases,54,55 but significant changes in cyclin-dependent kinases could not be detected by Western blot analysis and flow cytometric analysis in CD44hiCD8+ T cells after IL-15 and TGF-β treatment (data not shown). Because cytokine control is essential in CD8+ memory T-cell homeostasis, and the dominant phenotype of the DNRII transgenic CD8+ T cells was an increase in CD44hiCD122hi T cells, the role of TGF-β regulation of the IL-2/IL-15Rβ was investigated in nontransgenic CD8+ T cells. These studies demonstrated a profound negative effect of TGF-β on expression levels of the β-chain for the IL-15R complex after in vitro IL-15 stimulation at all time points examined and a subsequent negative effect on IL-15-induced CD8+ T-cell proliferation, suggesting that TGF-β functions, at least in part, as a negative cytokine regulator through cytokine receptor modulation. This is in contrast to some human studies that have shown IL-15 stimulation of CD8+ T cells to be resistant to TGF-β56 or to react in concert with TGF-β57-59 ; however, all these studies used preactivated CD8+ T cells that might have responded differently because of the previous activation or because of species differences. Although cytokine receptor modulation by TGF-β offers one potential mechanism for the changes observed in CD8+ memory T-cell growth in DNRII transgenic mice, other regulatory mechanisms are possible.2 Together, these data demonstrate that CD8+ memory T-cell homeostasis is regulated primarily negatively by TGF-β and that this negative regulation constitutes a critical and dominant tumor-suppressor pathway26 needed to oppose IL-15-driven proliferation of the CD8+ memory T-cell compartment, thus maintaining T-cell homeostasis and preventing potential malignant transformation. In addition, the modulation of IL-2/IL-15Rβ by TGF-β represents an observation with intriguing possibilities as a mechanism with respect to cytokine regulation of other cell subpopulations.
Prepublished online as Blood First Edition Paper, June 20, 2006; DOI 10.1182/blood-2006-05-025676.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Richard Hodes and John Letterio for helpful discussion and advice on the manuscript; Sue Sharrow, Veena Kapoor, and the Experimental Immunology Branch flow cytometry laboratory for technical advice and discussion; and Toby Gard-Weiss, Baishakhi Choudhury, Shafiqul Haq, Jianming Li, and Nyana Singh for excellent technical support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal