Abstract
Granulocyte colony-stimulating factor (G-CSF) is the principal cytokine regulating granulopoiesis. Truncation mutations of the G-CSF receptor (G-CSFR) are associated with the development of acute myeloid leukemia in patients with severe congenital neutropenia. Although increased proliferative signaling by a representative G-CSFR truncation mutation (termed d715) has been documented, the molecular basis for this hyperproliferative phenotype has not been fully characterized. Given the accumulating evidence implicating Src family kinases in the transduction of cytokine receptor signals, the role of these kinases in the regulation of G-CSF signaling was examined. We show that Hck and Lyn, Src family kinases expressed in myeloid cells, are negative regulators of granulopoiesis that act at distinct stages of granulocytic differentiation. Whereas Hck regulates the G-CSF-induced proliferation of granulocytic precursors, Lyn regulates the production of myeloid progenitors. Interestingly, d715 G-CSFR myeloid progenitors were resistant to the growth-stimulating effect of treatment with a Src kinase inhibitor. Together, these data establish Lyn and Hck as key negative regulators of granulopoiesis and raise the possibility that loss of Src family kinase activation by the d715 G-CSFR may contribute to its hyperproliferative phenotype.
Introduction
Maintenance of neutrophil homeostasis in the blood is critical to the regulation of the innate immune response and control of tissue inflammation.1 The level of circulating neutrophils is controlled primarily by the release of mature neutrophils from the bone marrow to blood and by the regulation of neutrophil production in the bone marrow. The latter process, termed granulopoiesis, is in turn regulated at several developmental stages, from the commitment of multipotential progenitors to the myeloid lineage through the terminal differentiation of granulocytic precursors. Granulocyte colony-stimulating factor (G-CSF) is widely used in the clinical setting to stimulate granulopoiesis. It modulates the proliferation and terminal differentiation of granulocytic precursor cells.2 In addition, recent data show that G-CSF regulates the production of myeloid lineage-committed progenitors.3 The importance of G-CSF in the regulation of granulopoiesis was confirmed by the characterization of mice deficient in G-CSF or G-CSF receptor (G-CSFR).4,5 These mice display chronic neutropenia with a decrease in granulocytic precursors and myeloid progenitors in the bone marrow. Recently, dominant-negative mutations of the G-CSFR have been implicated as the cause of impaired granulopoiesis in a subset of patients with severe congenital neutropenia.6-8 Together, these observations establish G-CSF as the key cytokine regulating basal granulopoiesis.
All of the biologic activities of G-CSF are mediated by the G-CSFR. This receptor lacks intrinsic kinase activity; instead, it recruits and activates several cytoplasmic tyrosine kinases, including members of the Janus kinase (JAK) and Src kinase families.9 Whereas the contribution of JAKs to G-CSFR signaling is established, the importance of Src kinases is less clear. On ligand binding, the G-CSFR has been shown to bind to and activate Lyn and Hck, 2 members of the Src kinase family.10,11 Lyn has been implicated in the activation of PI-3 kinase, the transmission of proliferative signals by G-CSF, and G-CSF-induced production of reactive oxygen species.12-14 Hck has been implicated in the transduction of phagocytic signals by G-CSF.15 Importantly, mice deficient for Lyn, Hck, and Fgr, the major Src family kinases expressed in myeloid cells, have normal resting granulopoiesis, though specific defects in mature neutrophil function are present.16,17 Recent studies suggest an inhibitory role for Lyn in the regulation of the myeloid lineage, specifically the monocyte/macrophage lineage.18 Aged lyn-/- mice exhibit an increased number of myeloid progenitors in their bone marrow and develop macrophage/monocyte tumors.
Altered G-CSF signaling has been implicated in the development of acute myeloid leukemia (AML) in patients with severe congenital neutropenia (SCN). SCN is a clinical syndrome manifested by severe neutropenia present at birth and a marked predisposition to develop AML or a myelodysplastic syndrome (MDS). Approximately 30% of patients with SCN acquire mutations of their G-CSFR.19 These mutations are almost invariably nonsense C>T transitions, resulting in the truncation of the distal cytoplasmic portion of the G-CSFR.20 These G-CSFR mutations are strongly associated with the development of AML. In the largest published study to date, 14 of 35 patients with G-CSFR mutations developed MDS or AML, whereas only 2 of 63 patients without G-CSFR mutations developed leukemia.21 We and others have generated transgenic mice carrying targeted mutations of their G-CSFR reproducing the truncations mutations found in patients with SCN.22,23 These mice display a markedly exaggerated neutrophil response to G-CSF treatment that is secondary to an increased number of myeloid progenitors and enhanced proliferation of granulocytic precursors. Despite this enhanced G-CSF responsiveness, no cases of AML or MDS have been detected in the G-CSFR mutant mice. Taken together, these data suggest that the G-CSFR mutations found in a subset of patients with SCN, while not sufficient, may contribute to leukemogenesis.
In this study, the contribution of Src family kinases to the regulation of granulopoiesis and transduction of G-CSF signals is characterized. We show that Hck and Lyn are negative regulators of granulopoiesis that act at distinct stages of granulocytic differentiation. Whereas Hck regulates the G-CSF-induced proliferation of granulocytic precursors, Lyn regulates the production of myeloid progenitors. Finally, evidence is provided that loss of Src family kinase activation by SCN-related G-CSFR mutations may contribute to their hyperresponsiveness to G-CSF.
Materials and methods
Mice
The generation of hck-/-, lyn-/-, fgr-/-, triple mutant hck-/- × lyn-/- × fgr-/- (HLF) and d715 G-CSFR mice has been previously described.16,23-25 All mice were backcrossed at least 10 generations onto a C57BL/6 background and were housed in a specific pathogen-free environment. Mice were sex- and age-matched (2-4 months) in all experiments. The Washington University Animal Studies Committee approved all experiments.
In vitro granulocytic differentiation assay
Bone marrow cells were harvested from mice 48 hours after treatment with 150 mg/kg 5-fluoruracil (5-FU; Sigma, St Louis, MO) and lineage-negative (Lin-) cells purified using the Miltenyi magnetic bead separation system, as described by the manufacturer (Miltenyi Biotec, Auburn CA). The following lineage antibodies were used: Gr-1, CD3, B220, and Ter-1119 (all antibodies from PharMingen, San Diego, CA). Where indicated, c-kit+ Lin- cells were directly purified from the bone marrow, as described (see “Flow cytometry”). A total of 1 × 104 Lin- or c-kit+ Lin- cells were cultured in α-minimum essential medium (α-MEM) containing 15% fetal calf serum (FCS), G-CSF (100 ng/mL; Amgen, Thousand Oaks, CA), and kit ligand (100 ng/mL; R&D Systems, Minneapolis, MN) in a humidified chamber with 6% CO2 for 7 days. Cell counts and 200-cell manual leukocyte differentials were performed at the indicated times to assess cellular proliferation and differentiation. In some cultures, the selective Src kinase inhibitor PP2 (Calbiochem, San Diego, CA) diluted in dimethyl sulfoxide (DMSO) was added at the indicated concentration and times. Control cultures containing an equal amount of DMSO were analyzed alongside the PP2-treated cultures.
Peripheral-blood analysis
Blood was obtained by retro-orbital venous plexus sampling in polypropylene tubes containing EDTA. Complete blood counts were determined using a Hemavet automated cell counter (CDC Technologies, Oxford, CT).
Hematopoietic progenitor-cell assays
Bone marrow was harvested by flushing both femoral bones with α-MEM containing 10% FCS. Bone marrow cells (5-10 × 104) were plated in 2.5 mL methylcellulose media (MethoCult 3230; StemCell Technologies, Vancouver, BC, Canada) supplemented with human G-CSF (100 ng/mL) or murine granulocyte-macrophage CSF (GM-CSF; 20 ng/mL, R&D Systems) and placed in a humidified chamber with 6% CO2. Colonies containing at least 50 cells were scored on day 7 to 10 of culture.
Flow cytometry
The c-Kit+ Lin- Sca+ (KLS) cells in the bone marrow were quantified as previously described.3 In brief, bone marrow cells were stained with fluorescein isothiocyanate (FITC)-conjugated Sca-1, allophycocyanin (APC)-conjugated c-Kit, and the following panel of phycoerythrin (PE)-conjugated lineage antibodies: CD2, B220, Gr-1, and Ter-119 (all antibodies from PharMingen). To sort c-Kit+ Lin- cells, bone marrow cells were stained with APC-conjugated c-Kit and the panel of PE-conjugated lineage markers. Cells were sorted and analyzed using a high-speed flow cytometer (Moflo-MLS; Cytomation, Fort Collins, CO).
Administration of G-CSF to mice
Recombinant human G-CSF diluted in PBS with 0.1% low endotoxin bovine serum albumin (Sigma) was administered by daily subcutaneous injection at a dose of 10 μg/kg/d for 7 days. Peripheral blood was obtained before the first G-CSF dose and 4 hours after injection on days 3, 5, and 7.
Electrophoretic mobility shift assay
Bone marrow cells (1 × 107) were resuspended in Opti-MEM 1 media (Gibco BRL, Grand Island, NY) with 1% FCS and stimulated at 37°C with buffer alone or G-CSF (100 ng/mL) for the indicated period. Nuclear extracts were prepared as previously described.26 Nuclear extract (1 μg) was incubated for 20 minutes at room temperature with 25 000 cpm 32P-labeled double-stranded oligonucleotide probe and 1 μg poly (dI:dC) in 20 μL binding buffer (10 mM Tris, pH 7.5, 100 mM KCL, 5 nM MgCL, 10% glycerol, and 1 mM DTT). Complexes were resolved on a 5% nondenaturing polyacrylamide gel. The gel was subsequently dried and analyzed by autoradiography. The following double-stranded oligonucleotides were used: m67 (5′-CATTTCCCGTAAATCAT-3′), a high-affinity mutant of the sis-inducible element of the human c-fos gene (SRF) and β-casein (5′-GATTTCTAGGAATTCAATCC-3′), the STAT-5-binding site of the bovine β-casein promoter. For supershift analysis, nuclear extracts were preincubated for 1 hour at 4°C with specific antibodies to STAT-3 (no. ST3-5G7; Zymed, San Francisco, CA) or STAT-5 (sc-835; Santa Cruz Biotechnology, Santa Cruz, CA) prior to the addition of radiolabeled oligonucleotide.
Immunoblotting
The c-Kit+ Lin- cells were cultured for 5 days in α-MEM with 15% FCS containing 100 ng/mL kit ligand and G-CSF. After starvation for 4 hours in Opti-MEM with 1% FCS, cells were stimulated for the indicated time with 100 ng/mL G-CSF. Cells were lysed in RIPA buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1 mM EDTA, 0.5% sodium deoxycholate, 1% NP40, 0.1% SDS, 0.5 mM PMSF, 0.5 mM diisopropyl fluorophosphate, and 10 μg/mL aprotinin and leupeptin) and immunblotted with antibodies directed against phospho-Erk-1/Erk-2 or pan-Erk-1/Erk-2 (9101S and 9102, respectively; Cell Signaling Technology, Beverly, MA).
Statistical analysis
Data are presented as mean ± SEM. Statistical significance was assessed using a 2-sided Student t test.
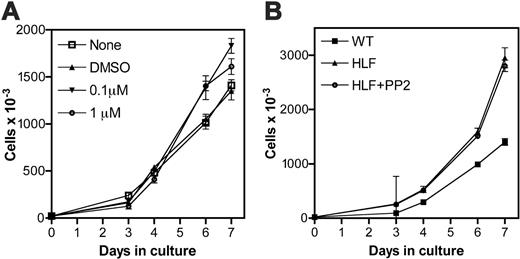
G-CSF-induced proliferation of primary myeloid progenitors. (A) 20 000 Lin- bone marrow cells from wild-type mice were cultured for 7 days in the presence of G-CSF and kit ligand. On day 3 of culture, the indicated amount of PP2 or vehicle alone (DMSO) was added to the culture. (B) 20 000 Lin- bone marrow cells isolated from wild-type (WT) or HLF mice were cultured for 7 days in the presence of G-CSF and kit ligand. On day 3 of culture, 1.0 μM PP2 was added to the indicated HLF culture. Treatment of HLF cells with vehicle alone (DMSO) had no effect on cell proliferation (data not shown). Data represent the mean ± SEM of triplicate measurements and are representative of 3 independent experiments.
G-CSF-induced proliferation of primary myeloid progenitors. (A) 20 000 Lin- bone marrow cells from wild-type mice were cultured for 7 days in the presence of G-CSF and kit ligand. On day 3 of culture, the indicated amount of PP2 or vehicle alone (DMSO) was added to the culture. (B) 20 000 Lin- bone marrow cells isolated from wild-type (WT) or HLF mice were cultured for 7 days in the presence of G-CSF and kit ligand. On day 3 of culture, 1.0 μM PP2 was added to the indicated HLF culture. Treatment of HLF cells with vehicle alone (DMSO) had no effect on cell proliferation (data not shown). Data represent the mean ± SEM of triplicate measurements and are representative of 3 independent experiments.
Results
Src kinases contribute to the proliferation, but not terminal granulocytic differentiation, of primary murine myeloid progenitors
We previously described an assay to study the terminal granulocytic differentiation of primary murine myeloid progenitors.26 In brief, bone marrow cells were harvested from 5-FU-treated mice, Lin- cells (enriched for progenitors) isolated, and the cells cultured for 7 days in the presence of kit ligand and G-CSF. In cultures of wild-type cells, near synchronous waves of granulocytic differentiation are observed, such that by day 7 of culture, more than 50% of cells are mature neutrophils. Using this system, the contribution of Src kinases to terminal granulocytic differentiation was assessed. When treated with PP2, a selective Src kinase inhibitor,27 a modest but reproducible increase in cellular proliferation was noted (Figure 1A). To confirm this observation, we next studied hematopoietic progenitors derived from mice lacking Hck, Lyn, and Fgr, the major Src kinases expressed in myeloid cells.16 As shown in Figure 1B, triple mutant hck-/- × lyn-/- × fgr-/- (HLF) progenitor cells demonstrated an enhanced proliferative response to G-CSF and kit ligand as compared to wild-type cells. Importantly, treatment with 1 μM PP2 did not further affect the expansion of HLF cultures, confirming that Hck, Lyn, and Fgr are the major Src kinases present in myeloid cells.
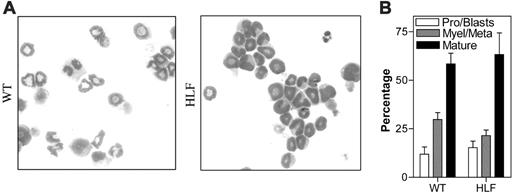
We next examined granulocytic differentiation in these cultures. As reported previously, approximately 60% of cells present on day 7 of culture of wild-type cells exhibited a mature neutrophil morphology (Figure 2).26 A similar percentage of mature neutrophils was present in cultures of HLF cells. Furthermore, addition of PP2 or vehicle alone to wild-type cultures had no impact on differentiation (data not shown). Collectively, these data show that one or more Src kinases act as negative regulators of granulocytic-cell proliferation but are not required for terminal granulocytic differentiation.
G-CSF-induced granulocytic differentiation of primary myeloid progenitors. WT and HLF Lin- bone marrow cells were cultured for 7 days in the presence of G-CSF and kit ligand. (A) Representative Wright-Giemsa stains of cytospin preparations from cells on day 7 of culture. (B) Pooled results from 3 independent experiments of manual leukocyte differentials performed on cells on day 7 of culture. Pro indicates promyelocytes; Myle, myelocytes; Meta, meta-myelocytes; Mature, segmented, ring, and band neutrophils. Data represent the mean ± SEM. All images were obtained using a Nikon Eclipse E600 microscope equipped with a Nikon Plan Apo 100×/1.40 numeric aperture oil objective (Nikon USA, Melville, NY). The microscope was equipped with a Sony DXC S500 digital camera (Sony Electronics, Park Ridge, NJ), and images were captured using Kodak Imaging for Windows software (Eastman Software, Billerica, MA).
G-CSF-induced granulocytic differentiation of primary myeloid progenitors. WT and HLF Lin- bone marrow cells were cultured for 7 days in the presence of G-CSF and kit ligand. (A) Representative Wright-Giemsa stains of cytospin preparations from cells on day 7 of culture. (B) Pooled results from 3 independent experiments of manual leukocyte differentials performed on cells on day 7 of culture. Pro indicates promyelocytes; Myle, myelocytes; Meta, meta-myelocytes; Mature, segmented, ring, and band neutrophils. Data represent the mean ± SEM. All images were obtained using a Nikon Eclipse E600 microscope equipped with a Nikon Plan Apo 100×/1.40 numeric aperture oil objective (Nikon USA, Melville, NY). The microscope was equipped with a Sony DXC S500 digital camera (Sony Electronics, Park Ridge, NJ), and images were captured using Kodak Imaging for Windows software (Eastman Software, Billerica, MA).
Hck negatively regulates the proliferation of granulocytic precursors in response to G-CSF
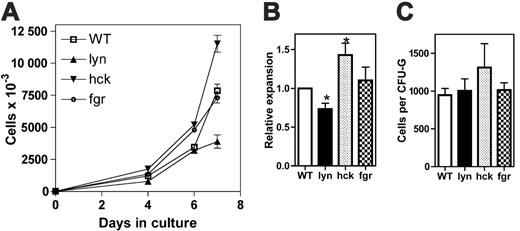
To determine the contribution of individual Src kinases to granulocytic differentiation, hematopoietic progenitor cells isolated from mice with single deletions of hck, lyn, or fgr were analyzed. To ensure a more uniform starting population, c-kit+ Lin- cells were used in this set of experiments. Interestingly, hck-/- progenitors displayed enhanced proliferation in response to G-CSF and kit ligand, whereas lyn-/- progenitors exhibited decreased proliferation (Figure 3). Fgr-deficient progenitors displayed normal granulocytic-cell proliferation. Granulocytic differentiation was similar in all cultures (data not shown). Because both G-CSF and kit ligand are required in this assay to support a reasonable level of granulocytic-cell proliferation, we examined G-CSF-induced growth in methylcellulose-based cultures. This is important because Lyn has been implicated in the regulation of proliferative signals by c-kit.28-30 Indeed, whereas a trend (nonsignificant) to increased colony size was observed in cultures of hck-/- cells, no difference was observed with lyn-/- cells. Of note, a similar number of colonies was observed in all of the cultures (data not shown), suggesting that on a per-cell basis, the number of granulocyte colony-forming units (CFU-Gs) per c-kit+ Lin--cell was similar for each genotype. Collectively, these data suggest that Lyn is not required for the efficient transduction of proliferative signals by G-CSF in primary granulocytic cells. Conversely, Hck is a negative regulator of G-CSF-induced proliferation in this cell population.
Lyn negatively regulates the number of committed myeloid progenitors and multipotential progenitors in the bone marrow
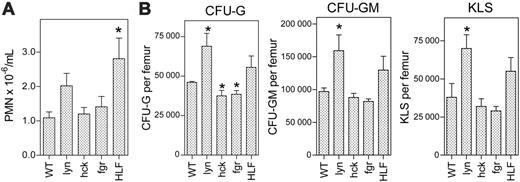
To assess the contribution of Src kinases to the regulation of granulopoiesis in vivo, we first measured the number of mature neutrophils in the blood of HLF, lyn-/-, hck-/-, and fgr-/- mice. Consistent with previous reports,18,25 the basal level of circulating neutrophils was similar in all groups of mice except HLF mice where a modest increase was observed (Figure 4). We next assessed the number of myeloid progenitors in the various Src kinase family-deficient mice (Figure 4). The number of granulocyte (CFU-G) and granulocyte-macrophage (CFU-GM) colonies in the bone marrow was significantly increased in lyn-/- mice. Interestingly, the number of multipotential progenitors, measured by flow cytometry as c-Kit+Lin- Sca+ (KLS) cells, also was significantly increased in lyn-/- mice. Of note, the increase in myeloid progenitors and KLS cells is secondary to their increased frequency in the bone marrow, because bone marrow cellularity in lyn-/- mice was comparable to wild-type mice (nucleated cells × 106/per femur: 24.5 ± 2.4 versus 23.0 ± 1.0, respectively; P = NS). A similar trend was observed in HLF mice. In contrast, the number of CFU-Gs was modestly but significantly reduced in hck-/- and fgr-/- mice. These data suggest that Src kinases regulate the production or survival of myeloid-committed progenitors in the bone marrow, with Lyn playing a dominant and inhibitory role.
G-CSF-dependent proliferation of individual src family-deficient progenitors. (A-B) c-Kit+ Lin- cells were isolated from the bone marrow of mice and cultured for 7 days in the presence of G-CSF and kit ligand (n = 3-5, each genotype). (A) Representative experiment. Data represent the mean ± SEM of triplicate measurements. (B) The fold expansion of cells in culture on day 7 is shown. In each experiment, the fold expansion of wild-type cells was defined as 1. (C) c-Kit+ Lin- cells were placed into methylcellulose containing 100 ng/mL G-CSF and cultured for 7 days (n = 3). Shown is the average number of cells per colony on day 7 of culture. A total of 150 to 282 colonies were analyzed for each genotype. Data represent the average ± SEM. *P < .05.
G-CSF-dependent proliferation of individual src family-deficient progenitors. (A-B) c-Kit+ Lin- cells were isolated from the bone marrow of mice and cultured for 7 days in the presence of G-CSF and kit ligand (n = 3-5, each genotype). (A) Representative experiment. Data represent the mean ± SEM of triplicate measurements. (B) The fold expansion of cells in culture on day 7 is shown. In each experiment, the fold expansion of wild-type cells was defined as 1. (C) c-Kit+ Lin- cells were placed into methylcellulose containing 100 ng/mL G-CSF and cultured for 7 days (n = 3). Shown is the average number of cells per colony on day 7 of culture. A total of 150 to 282 colonies were analyzed for each genotype. Data represent the average ± SEM. *P < .05.
Lyn is a negative regulator of G-CSF-stimulated granulopoiesis in vivo
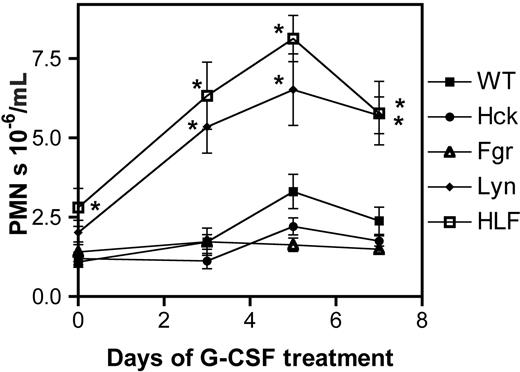
To further characterize the role of Src kinases in G-CSFR signaling, we measured the neutrophil response in the various Src family kinase-deficient mice after low-dose G-CSF treatment (10 μg/kg/d for 7 days; Figure 5). At this dose of G-CSF, neutrophil number in the blood was only modestly increased in wild-type mice (2.8 ± 0.64-fold increase on day 5 of G-CSF treatment relative to baseline). Similar increases were observed in hck-/- and fgr-/- mice (3.0 ± 0.68 and 2.6 ± 0.70, respectively). However, in HLF and lyn-/- mice, a markedly accentuated neutrophil response was observed (8.7 ± 3.9 and 7.8 ± 2.8, respectively). These data demonstrate that Lyn is a potent negative regulator of G-CSF-stimulated granulopoiesis in vivo.
Analysis of basal granulopoiesis. (A) The number of neutrophils in the blood was measured in 10 to 15 mice of each genotype. (B) Progenitor analyses. The number of CFU-G, CFU-GM, and KLS cells per femur was determined in 4 to 5 mice of each genotype. All mice were analyzed at baseline. The data represent the mean ± SEM. *P < .05 compared with wild-type mice.
Analysis of basal granulopoiesis. (A) The number of neutrophils in the blood was measured in 10 to 15 mice of each genotype. (B) Progenitor analyses. The number of CFU-G, CFU-GM, and KLS cells per femur was determined in 4 to 5 mice of each genotype. All mice were analyzed at baseline. The data represent the mean ± SEM. *P < .05 compared with wild-type mice.
Inhibition of Src kinase activity does not enhance the G-CSF-induced proliferation of d715 G-CSFR progenitors
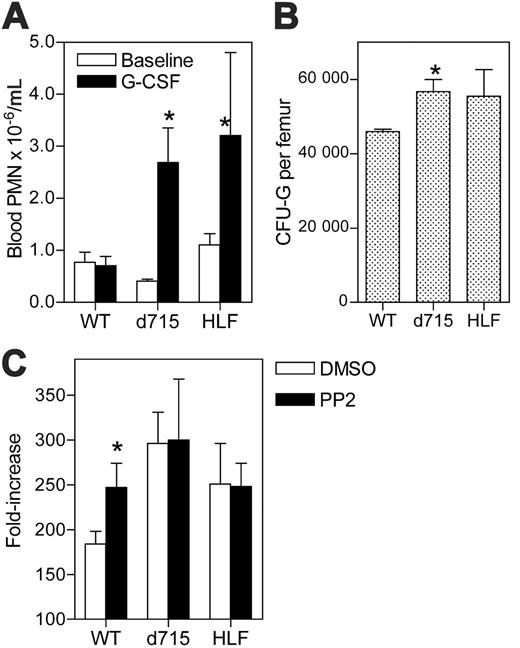
We previously described the generation of targeted transgenic mice carrying a mutated G-CSFR allele representative of those found in a subset of patients with SCN.23 Interestingly, these mice (termed d715 G-CSFR) display alterations in granulopoiesis that are similar to those observed in HLF mice. Specifically, both mice have normal (or near normal) blood-neutrophil counts but display an accentuated neutrophil response to G-CSF treatment (Figure 6A). Moreover, a similar increase in the number of CFU-Gs in the bone marrow was observed in both types of mice (Figure 6B).
Together with prior data suggesting that Lyn and Hck physically associate with and transduce signals from the G-CSFR,10,11 these observations raise the possibility that loss of Src kinases may contribute to the hyperproliferative phenotype of the d715 G-CSFR. To explore this possibility, we first determined whether treatment with PP2 augmented the proliferation of d715 myeloid progenitors in vitro. As reported previously, d715 progenitors display increased proliferation in vitro in response to G-CSF and kit ligand,26 similar to that observed with HLF progenitors (Figure 6C). However, unlike wild-type progenitors, no enhancement of proliferation with PP2 treatment was observed with d715 or HLF progenitors. These data show that d715 progenitors are resistant to PP2 treatment, suggesting that the hyperproliferative phenotype of the d715 G-CSFR may, in part, be secondary to its failure to activate Src kinases.
Src kinases contribute to the activation of STAT-3 and Erk by G-CSF
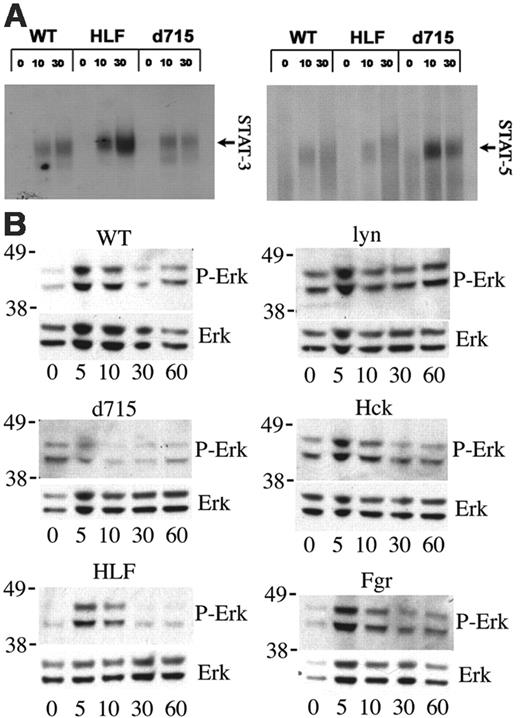
Previous studies have shown that STAT-5 activation by the d715 G-CSFR is accentuated.22,26 Because Src kinases have been implicated in the activation of STAT proteins,31,32 it is possible that loss of Src kinase activation by the d715 G-CSFR contributes to the altered STAT-5 activation. To test this hypothesis, we assessed STAT-3 and STAT-5 activation by G-CSF in bone marrow cells isolated from d715 G-CSFR and HLF mice. As reported previously, in primary hematopoietic cells, G-CSF activates STAT-3 and to a lesser degree STAT-5 (Figure 7A).22,33 Consistent with previous reports, d715 G-CSFR cells exhibit enhanced and prolonged STAT-5 activation by G-CSF, whereas STAT-3 activation was normal.22,26 The opposite was observed with HLF bone marrow cells; G-CSF-induced STAT-3 activation was consistently increased, whereas STAT-5 activation was normal.
Lyn has been implicated as negative regulator of mitogen-activated protein kinase (MAPK) cascades, in particular, Erk activation.34 G-CSF-induced Erk activation was assessed in cultured primary myeloid cells obtained from d715 G-CSFR or Src family kinase-deficient mice (Figure 7B). In wild-type cells, G-CSF induced a transient activation of Erk that peaked at 5 minutes. In contrast Erk activation in lyn-/- myeloid cells is enhanced at baseline and demonstrates prolonged G-CSF-induced activation. No consistent difference in G-CSF-induced Erk activation was observed in hck-/-, fgr-/-, or surprisingly, HLF cells. These data show that Lyn is a negative regulator of G-CSF-induced Erk activation in primary myeloid cells. In contrast, Erk activation by G-CSF was consistently reduced in d715 myeloid cells, demonstrating that enhanced Erk activation is not responsible for the hyperproliferative phenotype of the d715 G-CSFR.
In vivo neutrophil response to G-CSF. Mice (n = 10, each genotype) were treated with G-CSF (10 μg/kg/d for 7 days) and the number of neutrophils in the blood measured. Data represent the mean ± SEM. *P < .05 compared with wild-type mice at the same time point.
In vivo neutrophil response to G-CSF. Mice (n = 10, each genotype) were treated with G-CSF (10 μg/kg/d for 7 days) and the number of neutrophils in the blood measured. Data represent the mean ± SEM. *P < .05 compared with wild-type mice at the same time point.
Discussion
In this study, the contribution of Src family kinases to the regulation of granulopoiesis and G-CSF signaling were characterized. We first examined the contribution of Src kinases to the terminal differentiation and proliferation of granulocytic precursors. Myeloid progenitors lacking Hck, Lyn, and Fgr (the 3 major Src kinases expressed in myeloid cells) displayed enhanced cellular proliferation but normal terminal granulocytic differentiation. Similar results were observed with wild-type progenitors treated with PP2, a selective Src kinase family inhibitor. Surprisingly, analysis of myeloid progenitors from mice lacking individual Src kinases suggested antagonistic roles for Hck and Lyn in the regulation of granulocytic-cell proliferation. Whereas Lyn-deficient myeloid progenitors displayed impaired proliferation in response to G-CSF and kit ligand, the opposite was observed with Hck-deficient progenitors (Figure 3). Studies of progenitor growth in response to G-CSF alone confirmed a negative role for Hck in the transduction of proliferative signals by G-CSF. In contrast, G-CSF-induced proliferation of lyn-/- progenitors was comparable to wild-type cells. This result is somewhat surprising because previous studies had established that lyn plays an obligatory role in the transmission of proliferative signals by the G-CSFR in cell lines.12 However, the contribution of Lyn to G-CSF signaling may be dependent on the cellular context; whereas Lyn may play an important role in the transduction of proliferative signals in more primitive progenitors, its contribution in granulocytic precursors appears minimal. This result highlights the importance of studying proliferative responses in primary bone marrow cells rather than cell lines. Interestingly, both positive and negative roles for Lyn in the transduction of proliferative signals by c-kit in primary mast cells have been described.28,29 Whether these differences are related to the stage of mast-cell differentiation is not clear.
G-CSF responsiveness of d715 G-CSFR progenitors. (A) Mice (n = 4-5, each genotype) were treated with G-CSF (10 μg/kg/d for 7 days). Neutrophil number in the blood was quantified at baseline and on day 7. (B) The number of CFU-Gs per femur was determined in untreated mice (n = 4-5, each genotype; *P < .05 compared with wild-type mice). (C) c-Kit+ Lin- cells were isolated from mice (n = 3-8, for each genotype) and cultured in media containing G-CSF and kit ligand for 7 days in the presence of 1 μM PP2 or vehicle alone (DMSO). The fold increase in cell number on day 7 is shown. Data represent the mean ± SEM. *P < .05 compared with DMSO-treated mice of the same genotype.
G-CSF responsiveness of d715 G-CSFR progenitors. (A) Mice (n = 4-5, each genotype) were treated with G-CSF (10 μg/kg/d for 7 days). Neutrophil number in the blood was quantified at baseline and on day 7. (B) The number of CFU-Gs per femur was determined in untreated mice (n = 4-5, each genotype; *P < .05 compared with wild-type mice). (C) c-Kit+ Lin- cells were isolated from mice (n = 3-8, for each genotype) and cultured in media containing G-CSF and kit ligand for 7 days in the presence of 1 μM PP2 or vehicle alone (DMSO). The fold increase in cell number on day 7 is shown. Data represent the mean ± SEM. *P < .05 compared with DMSO-treated mice of the same genotype.
STAT and Erk activation. (A) Bone marrow cells were stimulated with G-CSF (100 ng/mL) for the indicated time (in minutes) and EMSA assays performed with oligonucleotide probes specific for STAT-3 (left panel) and STAT-5 (right panel). The identity of the complexes was confirmed by supershifting with appropriate antibodies (data not shown). (B) c-Kit+ Lin- cells were isolated from the bone marrow and cultured for 5 days in the presence of kit ligand and G-CSF to generate a cell population enriched in granulocytic precursors. After starvation for 4 hours in cytokine-free media, the cells were stimulated with G-CSF (100 ng/mL) for the indicated time (in minutes) and Western blot assays performed to measure phosphorylated Erk (P-Erk) or total Erk (Erk) protein. Molecular weight markers (in kDa) are shown at the left of each blot. Data are representative of 3 independent experiments.
STAT and Erk activation. (A) Bone marrow cells were stimulated with G-CSF (100 ng/mL) for the indicated time (in minutes) and EMSA assays performed with oligonucleotide probes specific for STAT-3 (left panel) and STAT-5 (right panel). The identity of the complexes was confirmed by supershifting with appropriate antibodies (data not shown). (B) c-Kit+ Lin- cells were isolated from the bone marrow and cultured for 5 days in the presence of kit ligand and G-CSF to generate a cell population enriched in granulocytic precursors. After starvation for 4 hours in cytokine-free media, the cells were stimulated with G-CSF (100 ng/mL) for the indicated time (in minutes) and Western blot assays performed to measure phosphorylated Erk (P-Erk) or total Erk (Erk) protein. Molecular weight markers (in kDa) are shown at the left of each blot. Data are representative of 3 independent experiments.
Previous studies demonstrated that loss of myeloid-expressed Src family kinases does not result in reduction in neutrophil levels in the peripheral blood.16,24,25 Although specific defects in neutrophil function were identified, these observations suggested that Src kinases were dispensable in the regulation of granulopoiesis in vivo. Of note, lyn-/- mice develop an age-dependent myeloproliferative disorder characterized by splenomegaly and monocyte/macrophage tumors.18 However, no increase in neutrophil production was observed in these mice. In the present study, we show that the level of circulating neutrophils is modestly but significantly increased in HLF mice with a trend toward an increase in lyn-/- mice (Figure 4A). This increase in circulating neutrophils is enhanced after G-CSF treatment, achieving statistical significance in both HLF and lyn-/- mice (Figure 5). At first glance, these data seem at odds with our in vitro studies of G-CSF-induced proliferation of myeloid progenitors. A potential explanation is provided by the finding that the number of G-CSF responsive progenitors (CFU-Gs) in the bone marrow of Lyn-deficient mice is increased, whereas a modest decrease was observed in Hck-deficient mice (Figure 4B). Thus, the normal basal neutrophil count in Hck-deficient mice might reflect a balance between a reduced number of CFU-Gs but an increase in their proliferative capacity. Conversely, the increased neutrophil response to G-CSF treatment in lyn-/- mice is likely due, in least in part, to the increased number of CFU-Gs in the bone marrow. Finally, in HLF mice, in which both the number of CFU-Gs and their proliferative capacity is increased, the greatest neutrophil response to G-CSF is seen. Interestingly, lyn-/- mice also have a 2-fold increase in KLS cells, suggesting that Lyn regulates the production of more primitive hematopoietic progenitor cells. The latter observation is consistent with a prior study showing that lyn-/- mice have increased numbers of myeloid and erythroid progenitors in their spleen and blood.35 Collectively, these data suggest that Lyn is the dominant Src kinase regulating granulopoiesis in vivo and acts predominantly by negatively regulating the production or survival of myeloid progenitors.
Truncation mutations of the G-CSFR are found in a subset of patients with SCN and are strongly associated with the development of leukemia. We and others have previously shown that transgenic mice expressing a representative G-CSFR mutant (termed d715 G-CSFR) are hyperresponsive to G-CSF both in vivo and in vitro.22,23 Given its potential contribution to leukemogenesis, considerable effort has been directed at elucidating the molecular mechanisms underlying this hyperresponsiveness. At least 2 important internalization motifs are deleted in the d715 G-CSFR, resulting in delayed receptor internalization. Compared with wild-type G-CSFR, G-CSF-induced activation of STAT-5 and Akt by the d715 G-CSFR is prolonged.22,26,36 In addition, evidence indicates that activation of certain negative regulators of G-CSFR signaling by the d715 G-CSFR is impaired. Specifically, activation of the tyrosine phosphatases SHP1 and SHP2 and the inositol phosphatase SHIP1 by the d715 G-CSFR is impaired.37,38 Finally, in an elegant study, van de Geijn and colleagues showed that the d715 G-CSFR fails to efficiently induce SOCS3 expression and recruitment to the G-CSFR, leading to enhanced STAT-5 activation.39 In the present study, we show that HLF and d715 G-CSFR mice display a similarly enhanced neutrophil response to G-CSF treatment (Figure 6A). Moreover, in both groups of mice the number of myeloid progenitors in their bone marrow and the in vitro proliferative response of myeloid progenitors to G-CSF were increased (Figure 6B). These observations prompted us to explore whether loss of Src kinase activation might contribute to the hyperresponsiveness of the d715 G-CSFR. Previous studies have reported that Hck and Lyn directly associate with or are activated by the wild-type G-CSFR in myeloid-cell lines.10,11 Unfortunately, despite extensive efforts, we were unable to reproducibly detect such an interaction in primary murine myeloid cells. Instead, we compared the effect of PP2, a selective Src kinase inhibitor, on the in vitro proliferation of wild-type and d715 G-CSFR progenitors. In contrast to wild-type cells, d715 G-CSFR progenitors were insensitive to PP2 treatment, suggesting that Src kinase activation by the d715 G-CSFR is defective (Figure 6C). Specifically, these data suggest that Hck activation by the d715 G-CSFR is impaired because Hck appears to be the major Src kinase regulating the G-CSF-induced proliferation of granulocytic precursors. Consistent with this conclusion, Santini et al recently reported that G-CSF-dependent activation of Syk, which is thought to be dependent on Hck activation, also is impaired by the d715 G-CSFR.15 Collectively, these data suggest that loss of Hck activation by the d715 G-CSFR may contribute to its hyperproliferative phenotype. Whether Lyn activation by the d715 G-CSFR also is impaired and contributes to the increased production/survival of myeloid progenitors is an open question. Consistent with this possibility, the number of G-CSF-responsive progenitors (CFU-Gs) in the bone marrow is increased in both lyn-/- and d715 G-CSFR mice (Figures 4B and 6B). On the other hand, an increase in multipotential KLS cells was observed only in lyn-/- mice (number of KLS cells per femur: 23 836 ± 4085 [d715 G-CSFR] and 70 066 ± 8595 [lyn-/- mice]; P < .01).
Several studies have examined the contribution of Src kinases in the transmission of cytokine signals. In particular, Lyn has been shown to negatively regulate CSF-1 and GM-CSF signaling in monocytes/macrophages.18 Lyn activation by these receptors results in phosphorylation of immunoreceptor tyrosine-based inhibitor motif (ITIM)-bearing proteins PIR-B and SIRP-1α and the adaptor protein Dok-2.18,35 These proteins in turn recruit SHP-1 and SHIP-1, leading to the attenuation of cytokine signaling.18,40 Of note, G-CSF stimulation of wild-type primary murine myeloid cells did not result in detectable phosphorylation of PIR-B, SIRP-1α, or Dok-1 (data not shown), suggesting that Src kinases may regulate G-CSF signaling through distinct mechanisms. Herein, we show that G-CSF stimulation of HLF bone marrow cells results in enhanced STAT-3 activation but normal STAT-5 activation (Figure 7A). Although STAT-3 activation has been associated with proliferative signals in some systems,41 transgenic mice with a myeloid-specific loss of STAT-3 display enhanced granulopoiesis.42 Thus, the contribution of increased STAT-3 activation to the hyperproliferative response of HLF cells to G-CSF is unclear. We also show that the basal level of Erk activation and G-CSF-induced Erk activation are increased in lyn-/- myeloid cells (Figure 7B). These data are consistent with a previous report showing increased thrombopoietin-induced proliferation and Erk activation in lyn-/- primary megakaryocytic cells.34 Interestingly, neither enhanced STAT-3 nor Erk activation was observed after stimulation of d715 bone marrow cells, suggesting that distinct pathways mediate its hyperproliferative response.
In summary, we present evidence to suggest that Src kinases play a negative role in the regulation of granulopoiesis and G-CSF signaling. There are stage-specific requirements for different Src kinases. Whereas Hck predominantly regulates the G-CSF-dependent proliferation of granulocytic precursors, Lyn plays a major negative role in regulating myeloid progenitor production. Loss of Hck, and possibly Lyn, activation by the d715 G-CSFR may contribute to the hyperproliferative phenotype of the d715 G-CSFR. The G-CSF signaling pathways regulated by Hck and Lyn are not fully understood and warrant further investigation.
Prepublished online as Blood First Edition Paper, June 13, 2006; DOI 10.1182/blood-2006-05-024307.
Supported by grants from the National Institutes of Health (PO1 CA10193 [D.C.L.] and K08 HL004546-05 [M.L.M.]) and by a Summer Undergraduate Research Fellowship funded by an Undergraduate Biological Sciences Education Program grant from the Howard Hughes Medical Institute to Washington University (C.H.M.).
C.H.M. and M.L.M. designed and performed research, collected and analyzed data, and wrote the paper; F.L. and J.W. designed and performed research; S.P. and C.A.L. designed and performed research and provided vital new reagents; and D.C.L. analyzed data and wrote the paper.
C.H.M. and M.L.M. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal