Abstract
CCAAT/enhancer binding proteins (C/EBPs) play critical roles in myelopoiesis. Dysregulation of these proteins likely contributes to the pathogenesis of myeloid disorders characterized by a block in granulopoiesis. In one such disease, acute promyelocytic leukemia (APL), a promyelocytic leukemia–retinoic acid receptor α (PML-RARα) fusion protein is expressed as a result of a t(15;17) chromosomal translocation. Treatment of PML-RARα leukemic cells with all-trans retinoic acid (ATRA) causes them to differentiate into mature neutrophils, an effect thought to be mediated by C/EBPs. In this study, we assess the potential for cooperativity between increased C/EBP activity and ATRA therapy. We demonstrate that although both C/EBPα and C/EBPϵ can significantly prolong survival in a mouse model of APL, they are not functionally equivalent in this capacity. We also show that forced expression of C/EBPα or C/EBPϵ in combination with ATRA treatment has a synergistic effect on survival of leukemic mice compared with either therapy alone.
Introduction
CCAAT/enhancer binding proteins (C/EBPs) are a family of transcription factors that regulate cell growth and differentiation. Two members of this family, C/EBPα and C/EBPϵ, are of critical importance in granulopoiesis. Disruption of the C/EBPα gene in mice results in the loss of production of neutrophils and eosinophils,1 whereas mice that lack C/EBPϵ generate neutrophils and eosinophils with abnormal function, gene regulation, and morphology.2-4
C/EBPα is the founding member of the bZIP class of DNA-binding proteins.5 Members of this family contain distinct N-terminal transactivation domains, C-terminal leucine-zipper dimerization domains, and basic DNA-binding regions.6,7 C/EBPα's basic region confers not only its ability to bind DNA but also its inhibition of E2F pathways.8,9 Previous studies have shown that the integrity of DNA binding, transactivation, and E2F inhibition is required for C/EBPα-dependent granulocytic differentiation.9-12 Although the functional domains required for C/EBPϵ activity have not been well characterized, C/EBPϵ's role in directing expression of myeloid-specific genes associated with terminal differentiation of granulocytes has been clearly demonstrated.2,13-15
Because C/EBPα and C/EBPϵ are required for normal granulocytic differentiation, alterations in expression or function of these proteins likely contribute to the pathogenesis of acute myeloid leukemia (AML), a disease characterized by an early block in granulopoiesis. Prior studies16,17 provide evidence that C/EBPα and C/EBPϵ may play a role in the pathogenesis of acute promyelocytic leukemia (APL), a subtype of AML in which a t(15;17) chromosomal translocation juxtaposes the promyelocytic (PML) gene to the retinoic acid receptor α (RARA) gene, creating an aberrant PML-RARα fusion protein.18
A unique characteristic of PML-RARα leukemic cells is their sensitivity to all-trans retinoic acid (ATRA).19 Treatment with ATRA induces remissions in patients with APL by causing the leukemic cells to differentiate into mature neutrophils.20 While the mechanism underlying the sensitivity of promyelocytes to ATRA is not completely understood, we and others21,22 have suggested that C/EBPs mediate the ATRA-induced maturation of APL cells.
In the present study, we explore the mechanism by which C/EBPs prolong survival in a murine model of APL. We also assess the potential for cooperativity between increased C/EBP activity and ATRA therapy. We demonstrate that both C/EBPα and C/EBPϵ significantly prolong survival; however, they are not functionally equivalent in this capacity. We also show that forced expression of C/EBPα or C/EBPϵ in combination with ATRA treatment has a synergistic effect on survival of leukemic mice compared with either therapy alone.
Study design
Plasmids
A rat C/EBPα cDNA (rC/EBPα) was generated by polymerase chain reaction (PCR) and cloned into the tamoxifen-inducible pBabepuro3:hb estrogen receptor* (pBP3:hbER*) to generate pBP3:rC/EBPα-ER. For generation of MIG-rC/EBPα-ER, the rC/EBPα-ER fragment was excised from pBP3:rC/EBPα-ER and cloned into the mouse stem cell virus–internal ribosomal entry site–green fluorescent protein (MSCV-IRES-GFP [MIG]) retroviral vector as a BamHI-HincII fragment into the BglII-HpaI sites in MIG. Construction of MIG-hC/EBPϵ-ER has been previously described.16 Briefly, human C/EBPϵ-ER was cloned into the EcoRI-HpaII sites in MIG as an EcoRI-SacI fragment. Point mutations were made in the DNA-binding domains of rC/EBPRα and hC/EBPϵ at positions R289 and R211, respectively, using the QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. Position R211 in C/EBPϵ is equivalent to C/EBPRα289. The resulting mutants, C/EBPϵR211A and C/EBPαR289A, were confirmed by DNA sequencing.
Cell culture
The 32Dcl3 cell line was modified to express high levels of the ecotropic receptor (32Dcl3-eco R), thereby facilitating retroviral transduction. These cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin G, 100 μg/mL streptomycin, and 5% X63Ag8–mouse interleukin-3 (mIL-3)–conditioned media. BOSC23 cells were maintained in DMEM supplemented with 10% heat-inactivated FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Freshly harvested leukemic no. 1111 cells23 from bone marrow and spleen of leukemic mice were cultured in stem cell media (Myelocult M5300; StemCell Technologies, Vancouver, BC, Canada) with 15% FBS, 5% IL-3–conditioned media, 0.4 mM glutamine, 100 U/mL penicillin G, 100 μg/mL streptomycin, 10 ng/mL rIL-3, 10 ng/mL IL-6, and 10 ng/mL stem cell factor.
Retroviral transduction
BOSC23 cells were transfected with retroviral constructs as previously described.24 Retroviral supernatants were collected and used to transduce leukemic bone marrow (#1111; 2 × 106 cells per well) and 32Dcl3 cells (100 000 cells per well) as previously described.16 After transduction, leukemic #1111 and 32Dc13 cells were sorted using the fluorescence-activated cell sorter (FACS) Vantage (Becton Dickinson, San Jose, CA). GFP-positive bone marrow cells were injected into sublethally irradiated mice, and GFP-positive 32Dcl3-eco R cells were maintained in culture in the presence or absence of 20 nM 4-hydroxytamoxifen (4-HT; Sigma, St Louis, MO). At 48-hour intervals, live 32Dc13 cells were counted using trypan blue exclusion, and the percentage of GFP-positive cells was assessed by flow cytometry.
Mice
Mice were bred and maintained at the University of California at San Francisco, and their care was in accordance with Institutional Animal Care and Use Committee guidelines. To examine C/EBPs in vivo, retrovirally transduced GFP-positive leukemic no. 1111 cells (50 000 cells per mouse) were injected into the lateral tail vein of sublethally irradiated (4.5 Gy) 6- to 8-week-old female FVB/N mice. Each group (C/EBPα, C/EBPϵ, or the mutant version of each) contained 20 mice, with 5 animals in each subgroup (placebo, 4-HT, ATRA, and 4-HT plus ATRA). Treatments (placebo, 25 mg 4-HT, and 10 mg ATRA) were administered to each subgroup as 21-day release, subcutaneous pellets (Innovative Research of America, Sarasota, FL).
Statistical analysis
Statistical analyses were performed in GraphPad Prism using the log-rank test or in Excel 2000 using the Student t test with 2-tailed distribution and unequal variance as appropriate.
Results and discussion
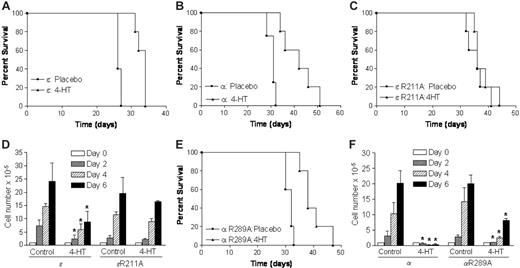
C/EBPα and C/EBPϵ play central roles in normal myelopoiesis; therefore, it is likely that altered function of these proteins contributes to the pathogenesis of APL. In a previous study,16 we showed that expression of a tamoxifen-inducible form of C/EBPϵ, hC/EBPϵ-ER, in leukemic cells caused them to differentiate into mature neutrophils in vivo. In the present study, we expand this approach to C/EBPα and assess the abilities of both C/EBPα and C/EBPϵ to prolong survival in a mouse model of APL. To assess the antileukemic effect of C/EBPs in this system, we transduced PML-RARα leukemic cells23 with either C/EBPα-ER or C/EBPϵ-ER retrovirus and transplanted them into sublethally irradiated histocompatible mice. After leukemias developed in the recipient animals, the mice were treated with either a placebo or 4-HT to induce C/EBP activity. In mice receiving transplants with C/EBPϵ-ER–transduced leukemias, treatment with 4-HT prolonged mean survival by 7 days compared with animals given a placebo (Figure 1A). Animals that received C/EBPα-ER–transduced leukemias demonstrated a more robust response following treatment with 4-HT (Figure 1B), exhibiting a mean increase in survival of 11 days compared with the placebo group. Therefore, tamoxifeninducible forms of both C/EBPα and C/EBPϵ significantly prolong survival in a mouse model of APL. Of note, 4-HT treatment of mice receiving untransduced cells had no effect on survival (data not shown).
Because C/EBPs belong to the bZIP family of DNA-binding proteins, we asked whether the ability to bind DNA was required for the antileukemic activities of C/EBPα and C/EBPϵ. To address this, we mutated R289 in C/EBPα and the corresponding amino acid (R211) in C/EBPϵ to alanine-generating mutants (C/EBPαR289A and C/EBPϵR211A) that were devoid of DNA-binding activity (data not shown). We selected these residues for substitution based on the observation that R289 is an integral component of the protein-DNA interface in the crystal structure of a C/EBPα bZIP polypeptide bound to its cognate DNA site.25 We then transduced PML-RARα leukemic cells with C/EBPαR289A or C/EBPϵR211A retrovirus and transplanted them as before. In animals receiving transplants with C/EBPϵR211A-transduced leukemias, we found that treatment with 4-HT had no effect on survival (Figure 1C), thus indicating that the DNA-binding activity of C/EBPϵ is required for its antileukemic effect. We observed a similar phenomenon in the factor-dependent myeloid progenitor cell line 32Dcl3 (Figure 1D). While the wild-type C/EBPϵ suppressed growth of 32Dc13 cells in the presence of 4-HT, the R211A mutant was unable to repress proliferation, suggesting that the DNA-binding activity of C/EBPϵ is required for its function in immature myeloid cells.
Interestingly, mice receiving transplants with C/EBPαR289A-transduced leukemias exhibited a mean increase in survival of 8 days compared with the placebo group following treatment with 4-HT (Figure 1E). This finding indicates that, unlike C/EBPϵ, C/EBPα can suppress leukemia without binding DNA. 32Dc13 cells transduced with either wild-type or mutant C/EBPα fail to proliferate following treatment with 4-HT, suggesting that C/EBPα has antiproliferative effects that do not require DNA binding (Figure 1F). Together, the results presented in Figure 1 imply that C/EBPα and C/EBPϵ are not functionally equivalent in their ability to suppress growth and inhibit leukemogenesis.
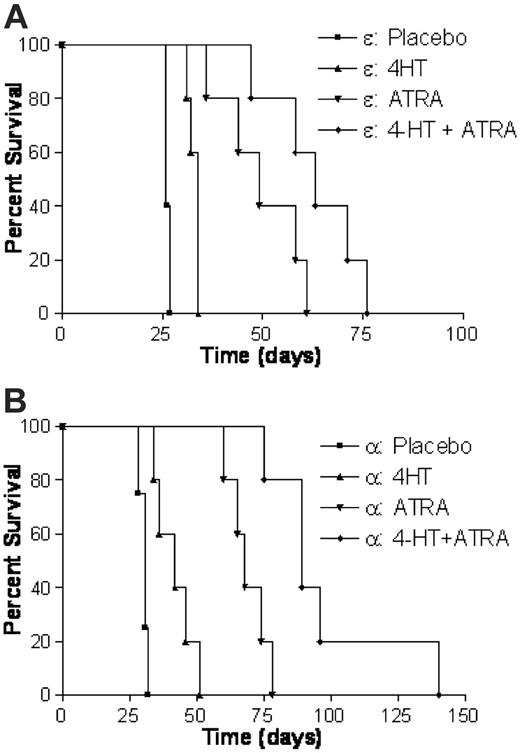
The results shown in Figure 1, in conjunction with the idea that C/EBPs mediate the antileukemic effects of ATRA, led us to assess the potential for cooperativity between treatment with ATRA and forced expression of C/EBPs. To address this relationship, we transduced PML-RARα leukemic cells with either C/EBPα-ER or C/EBPϵ-ER retrovirus and transplanted them as before. After leukemias developed in recipient animals, we treated them with either a placebo, 4-HT, ATRA, or a combination of 4-HT and ATRA. In mice receiving transplants with C/EBPϵ-ER–transduced leukemias, ATRA treatment resulted in a more substantial increase in survival (mean increase of 23 days compared with the placebotreated group) than did treatment with 4-HT (Figure 2A). However, treatment with a combination of 4-HT and ATRA had the greatest impact on survival, with a mean increase of 37 days compared with the placebo group.
Tamoxifen-inducible C/EBPs suppress growth of myeloid leukemias and 32Dc13 cells: DNA binding is requisite for the antiproliferative effects of C/EBPϵ but not C/EBPα. (A-C,E) Bone marrow of PML-RARα leukemic mice were harvested and transduced with retroviruses expressing C/EBPα-ER, C/EBPϵ-ER, or a mutant version of each that is deficient in DNA-binding activity (C/EBPαR289A-ER and C/EBPϵR211A-ER, respectively). Following 2 rounds of transduction, GFP-positive cells were sorted and injected into sublethally irradiated FVB/N females. After leukemias developed in recipient animals, 5 mice from each group were given either a placebo or 25 mg 4-HT. Animals that received C/EBPϵ-ER–transduced leukemias exhibited prolonged survival following treatment with 4-HT (mean survival time of 33 days compared with 26.4 days in the placebo group; P = .002). C/EBPϵR211A-ER had no effect on survival of leukemic mice (mean survival time of 37.4 days compared with 36.4 days in the placebo group). In animals receiving transplants with C/EBPα-ER– or C/EBPαR289A-ER–transduced leukemias, treatment with 4-HT significantly prolonged survival (P ≤ .003). Mean survival times for placebo and 4-HT–treated groups were 33.8 days and 46.4 days, respectively, for C/EBPα-ER mice and 31.4 days and 39.8 days, respectively, for C/EBPαR289A-ER mice. (D, F) 32Dcl3 cells were plated in 24-well dishes at 100 000 cells per well; transduced with retroviruses expressing C/EBPϵ-ER, C/EBPϵR211A-ER, C/EBPα-ER, or C/EBPαR289A-ER; and cultured in the absence or presence of 20 nM 4-HT. The growth curve represents the number of transduced cells at days 0, 2, 4, and 6. The results are mean ± SD from at least 3 independent experiments. (D) *P < .05 and (F) *P ≤ .01.
Tamoxifen-inducible C/EBPs suppress growth of myeloid leukemias and 32Dc13 cells: DNA binding is requisite for the antiproliferative effects of C/EBPϵ but not C/EBPα. (A-C,E) Bone marrow of PML-RARα leukemic mice were harvested and transduced with retroviruses expressing C/EBPα-ER, C/EBPϵ-ER, or a mutant version of each that is deficient in DNA-binding activity (C/EBPαR289A-ER and C/EBPϵR211A-ER, respectively). Following 2 rounds of transduction, GFP-positive cells were sorted and injected into sublethally irradiated FVB/N females. After leukemias developed in recipient animals, 5 mice from each group were given either a placebo or 25 mg 4-HT. Animals that received C/EBPϵ-ER–transduced leukemias exhibited prolonged survival following treatment with 4-HT (mean survival time of 33 days compared with 26.4 days in the placebo group; P = .002). C/EBPϵR211A-ER had no effect on survival of leukemic mice (mean survival time of 37.4 days compared with 36.4 days in the placebo group). In animals receiving transplants with C/EBPα-ER– or C/EBPαR289A-ER–transduced leukemias, treatment with 4-HT significantly prolonged survival (P ≤ .003). Mean survival times for placebo and 4-HT–treated groups were 33.8 days and 46.4 days, respectively, for C/EBPα-ER mice and 31.4 days and 39.8 days, respectively, for C/EBPαR289A-ER mice. (D, F) 32Dcl3 cells were plated in 24-well dishes at 100 000 cells per well; transduced with retroviruses expressing C/EBPϵ-ER, C/EBPϵR211A-ER, C/EBPα-ER, or C/EBPαR289A-ER; and cultured in the absence or presence of 20 nM 4-HT. The growth curve represents the number of transduced cells at days 0, 2, 4, and 6. The results are mean ± SD from at least 3 independent experiments. (D) *P < .05 and (F) *P ≤ .01.
Animals that received C/EBPα-ER–transduced leukemias demonstrated a similar pattern of response; however, the magnitude of each response was greater than that seen in C/EBPϵ-ER mice (Figure 1B). Treatment with 4-HT and ATRA prolonged the survival of C/EBPα-ER animals by a mean increase of 11 and 38 days, respectively, but the greatest impact on survival was seen in mice given the combined treatment (mean increase in survival was 67 days). The results presented in Figure 2 demonstrate that forced expression of C/EBPα or C/EBPϵ in combination with ATRA treatment has a synergistic effect on survival of leukemic mice compared with either therapy alone. These findings suggest that the ability of ATRA to prolong the survival of leukemic mice includes mechanisms beyond simple induction of C/EBP activity. Furthermore, and of potential clinical importance, our results indicate that agents that increase C/EBP activity might function cooperatively with ATRA to exert antileukemic activity in AML.
Forced expression of C/EBPs in combination with ATRA treatment has a synergistic effect on survival of leukemic mice. Leukemic animals expressing either C/EBPϵ-ER (A) or C/EBPα-ER (B) were generated as described in Figure 1 and treated with one of the following: a placebo, 25 mg 4-HT, 10 mg ATRA, or a combination of 4-HT and ATRA. The data for each C/EBP group are from 20 animals with 5 mice per treatment. The combined therapy of 4-HT and ATRA resulted in the most profound effect on survival in both groups (P < .001). The mean survival times of placebo, 4-HT, ATRA, and 4-HT plus ATRA groups were 26.4, 33, 49.6, and 63 days, respectively, in animals receiving transplants with C/EBPϵ-ER–transduced leukemias and 30.5, 41.8, 69, and 97.8 days, respectively, in C/EBPα-ER animals.
Forced expression of C/EBPs in combination with ATRA treatment has a synergistic effect on survival of leukemic mice. Leukemic animals expressing either C/EBPϵ-ER (A) or C/EBPα-ER (B) were generated as described in Figure 1 and treated with one of the following: a placebo, 25 mg 4-HT, 10 mg ATRA, or a combination of 4-HT and ATRA. The data for each C/EBP group are from 20 animals with 5 mice per treatment. The combined therapy of 4-HT and ATRA resulted in the most profound effect on survival in both groups (P < .001). The mean survival times of placebo, 4-HT, ATRA, and 4-HT plus ATRA groups were 26.4, 33, 49.6, and 63 days, respectively, in animals receiving transplants with C/EBPϵ-ER–transduced leukemias and 30.5, 41.8, 69, and 97.8 days, respectively, in C/EBPα-ER animals.
Prepublished online as Blood First Edition Paper, June 20, 2006; DOI 10.1182/blood-2006-02-003582.
Supported by National Institutes of Health (NIH) grants CA95274 (S.C.K) and CA095512-01 (D.P.). S.C.K. is a scholar of the Leukemia and Lymphoma Society and was a Burroughs-Wellcome Fund Career awardee.
Y.-J.L. designed and performed experiments and analyzed data; L.C.J. performed experiments, analyzed data, and wrote the manuscript; N.A.T. designed and performed experiments and edited the manuscript; D.P. provided reagents and edited the manuscript; D.G.T. mentored this line of investigation and edited the manuscript; and S.C.K. designed experiments, analyzed data, provided funding for this work, and edited the manuscript.
Y.-J.L. and L.C.J. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal