Abstract
Regulatory dendritic cells (DCs) play an important role in maintaining peripheral tolerance or immune homeostasis. Our previous study demonstrated that mature DCs could be driven by splenic stroma to proliferate and differentiate into a novel subset of regulatory DCs (diffDCs) displaying a Th2-biased cytokine profile. However, the underlying mechanisms for the unique cytokine profile of diffDCs and how diffDCs regulate the innate and adaptive immunity in response to toll-like receptor (TLR) agonists remain unclear. Here, we report that unlike immature DCs, diffDCs secrete more interleukin 10 (IL-10) but little IL-12p70 in response to lipopolysaccharide (LPS) or other TLR agonists. Up-regulation of extracellular signal-regulated kinase (ERK1/2) activation was shown to be responsible for IL-10 preferential production, and suppression of p38 activation was for impaired IL-12p70 production in diffDCs. Interestingly, LPS treatment could not reverse the inhibitory effect of diffDCs on the proliferation of antigen-specific CD4+ T cells. However, diffDCs could activate natural killer (NK) cells through diffDC-derived IL-10, and even more markedly after stimulation of TLR agonists. These diffDC-activated NK cells could in turn kill surrounding diffDCs. Our results illuminate signal pathways for the unique cytokine profile of diffDCs, and diffDCs can exert their regulatory function even after inflammatory stimuli, thus reflecting one way for strict regulation of immune response.
Introduction
Dendritic cells (DCs) are the most potent professional antigen-presenting cells (APCs) that integrate a wide array of incoming signals and convey them to lymphocytes, directing the appropriate immune responses.1 Induction of immune response or tolerance by DCs may be explained by DCs at different developmental stages with different functions, or existence of different subsets of DCs. Immature DCs (imDCs) have been shown to be able to induce tolerance.2 However, upon inflammatory stimulation or uptake of pathogenic antigens, imDCs migrate to secondary lymph organs undergoing maturation. Mature DCs (maDCs) have a potent immune-stimulatory function by production of cytokines such as interleukin 12 (IL-12) p70, up-regulation of costimulatory molecules, and potent ability to induce Th1 response and activate an antigen-specific CD8+ T-cell response.3
Recently, subsets of regulatory DCs were identified and considered to be important in maintaining immune homeostasis.4-6 Regulatory DCs negatively regulate immune response by inducing a generation of regulatory T (Treg) cells or a preferential Th2 response.7-9 During investigation of the fate of maDCs after antigen presentation and T-cell activation, we surprisingly found that splenic stromal cells, mimicking the secondary lymph organ microenviroment where maDCs present antigen to lymphocytes, could drive maDCs to proliferate and differentiate into a novel subtype of regulatory DCs (diffDCs), which inhibit rather than activate antigen-specific T-cell proliferation.10 Compared with fully matured DCs, diffDCs have a phenotype similar to that of imDCs. diffDCs also display a Th2-biased cytokine profile by secreting high IL-10 but little IL-12p70.10 However, the molecular mechanism of this Th2-biased cytokine production in diffDCs remains unclear.
Toll-like receptors (TLRs) expressed by APCs recognize a set of conserved pathogen-associated molecular patterns (PAMPs) and signal to the host the presence of pathogens. imDCs undergo maturation upon recognition of pathogenic components via TLRs, which enable DCs to produce a different cytokine profile and control the development of Th1/Th2-type adaptive immune responses.11 And yet, how diffDCs will respond to such TLR agonists has not been identified so far.
The functional diversity of DCs is in large part due to the many DC subsets and lineages in different organs or tissues.12-14 Three major DC subsets have been identified in mouse spleen, and more DC subsets are located in lymph nodes. Natural killer (NK) cells are also enriched in the spleen and some CD62L+CCR7+NK cells have been identified in lymph nodes.15-17 Moreover, maDCs could recruit peripheral NK cells to lymph nodes in a CXCR3-dependent manner, whereas human spleen DC-derived IL-12 and IL-15 play distinct roles in splenic NK-cell activation.18,19 These findings suggest that secondary lymphoid organs may be potential locations for DC–NK-cell interaction. However, whether a DC subset can activate or inhibit NK cells in these locations and what the consequence of these interactions may be have not been elucidated.
In this study, we report that diffDCs preferentially secrete IL-10 but not IL-12 through differential regulation of the ERK and p38 pathways. TLR agonists could not reverse this bias or the ability of diffDCs to inhibit proliferation of antigen-specific CD4+ T cells. Furthermore, partially through preferential IL-10 production, diffDCs enhance IFN-γ production and cytotoxicity of NK cells and the NK activation is even more significant after coculture with diffDCs stimulated with TLR agonists. These diffDC-activated NK cells could in turn kill surrounding diffDCs. In addition to their regulatory effects on T cells and NK cells, diffDCs, producing more IL-10 but less IL-12p70 even after TLR agonist stimulation, might play an important role in the strict regulation of immune response at the APC level in a feedback manner.
Materials and methods
Reagents and mice
Recombinant mouse granulocyte macrophage–colony-stimulating factor (GM-CSF) and IL-4 were purchased from PeproTech (London, United Kingdom). RPMI1640 and fetal bovine serum (FBS) were from Hyclone (Loga, UT). Lipopolysaccharide (LPS) (Escherichia coli, O26:B6), LTA, and polyI:C were purchased from Sigma (St Louis, MO). Nuclease-resistant phosphorothioate oligodeoxynucleotides were supplied by Sangon (Shanghai, China) and had no detectable endotoxins by Limulus assay. SB203 580, a p38 inhibitor; U0126, a mitogen-activated protein kinase kinase (MEK) inhibitor; SP600 125, a c-Jun NH2-terminal kinase (JNK) inhibitor; and pyrrolidinecarbodithoic acid (PDTC), an inhibitor of nuclear factor κB (NF-κB) were purchased from Calbiochem (San Diego, CA). Neutralizing monoclonal anti–mouse IL-10 antibody was purchased from R&D Systems (Minneapolis, MN). Anti-CD11c–FITC was purchased from PharMingen (San Diego, CA). Anti–phospho-ERK monoclonal antibody (mAb), anti-ERK polyclonal antibody, anti–phospho-p38 MAP kinase mAb, anti–phospho-JNK mAb, anti–NF-κBp65 (sc-109), anti–phospho-NF-κBp65, and their respective horseradish peroxidase–coupled secondary antibodies were purchased from Santa Cruz (Santa Cruz, CA). Anti–κB-α was produced by Biolabs (Beverly, MA). C57BL/6J mice at 5 to 6 weeks of age were obtained from Joint Ventures Sipper BK Experimental Animal (Shanghai, China). OVA323-339-specific TCR-transgenic mice (DO11.10) and IL-10 gene-disrupted mice were obtained from Jackson Laboratory (Bar Harbor, ME) and were bred in specific pathogen-free conditions.
Culture of mouse imDCs, maDCs, and regulatory DCs (diffDCs)
Bone marrow–derived DCs from different mice were generated as described previously.20 Briefly, bone marrow progenitors were cultured in 10 ng/mL GM-CSF and 1 ng/mL IL-4. Nonadherent cells were gently washed out on day 4 of culture; the remaining loosely adherent clusters were cultured for an additional 4 to 5 days in the presence of 10 ng/mL LPS. At day 8, maDCs were positively selected using CD11c magnetic microbeads (Miltenyi Biotec, Auburn, CA). The cells cultured only with the same concentrations of GM-CSF and IL-4 for 6 days were harvested as imDCs. diffDCs were generated as described previously.10 Once monolayers of endothelial-like splenic stromal cells (ESSCs) had reached 50% to 60% confluence, maDCs were seeded at a density of 2 × 106 per 5 mL/well in 6-well plates in RPMI1640 medium supplemented with 5% FCS for at least 7 days. diffDCs were washed off the layer using 0.1% trypsin and 5 mM EDTA, and purified using CD11c magnetic microbeads.
Assay for antigen presentation
Splenic CD4+ T cells from DO11.10 OVA323-339-specific TCR transgenic x C57BL/6 F1 hybrid mice were positively selected by MACS for use as antigen-specific responders, then cocultured with live DCs in the presence of OVA323-339 peptide (LCMV-NP309-328 was used as an Iab-restricted peptide control) at a ratio of 1:10 (DCs/T) in round-bottom 96-well plates (1 × 105 T cells/200μL/well) for 5 days. Cells double stained with anti-CD4–FITC and 7-amino-actinomycin D (7-AAD), resuspended in exactly 300 μL PBS and cellular data acquired for 56 seconds using a flow cytometer. The number of CD4+7-AAD– live cells was calculated to represent the altitude of antigen-specific CD4 T-cell proliferation.
Cytokine assays
Mouse IL-12p70 and IL-10 levels were assayed by enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (R&D Systems).
Western blotting
Cells were lysed with M-PER Protein Extraction Reagent (Pierce, Rockford, IL) supplemented with protease inhibitor cocktail, and protein concentrations of the extracts measured by BCA assay (Pierce). Proteins were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto nitrocellulose membranes, then blotted as described previously.21
Assessment of IκB-α degradation and NF-κB phosphorylation and nuclear translocation
Cytoplasmic and nuclear extracts were prepared using NE-PER nuclear and cytoplasmic extraction reagents (Pierce), and the protein concentration was determined by BCA-200 protein assay. IκB-α in cytoplasmic extracts and NF-κB subunit p65 in nuclear extracts were detected by Western blot using specific antibodies.
Isolation and purification of NK cells
Spleen-derived resting NK cells were enriched using DX5-conjugated microbeads as recommended by the manufacturer (Miltenyi Biotec). According to the manufacturer's recommendations, NK cells were used when more than 90% DX5 positive.
diffDC-NK coculture
Coculture experiments were performed with splenic NK cells and DCs from wild-type (WT) or IL-10–/–C57BL/6J mice. First, purified imDCs, maDCs, diffDCs, or TLR agonist–diffDCs were resuspended in RPMI1640 supplemented with 10% FCS and plated in 96-well U-bottom plates at a density of 1.0 × 105 per well. Then, freshly purified resting NK cells were seeded to DCs at the indicated ratios (DC/NK) in a total volume of 200 μL per well. In some cases, before coculture with NK cells, DCs were incubated with anti–IL-10 and/or anti–IL-12 neutralizing mAbs for 1 hour. In some experiments, IL-10–/–diffDCs or IL-10–/–LPS-diffDCs were cocultured with NK cells in the presence of exogenous IL-10 (Ebioscience, San Diego, CA) at the concentration of 300 pg/mL or 700 pg/mL, respectively. For intracellular staining, Brefeldin A (Sigma-Aldrich) was added to the coculture system at a final concentration of 10 μg/mL simultaneously. For analysis of DC lysis, activated NK cells were collected from conditioned diffDC–NK-cell coculture after 18 hours, then added into freshly isolated maDCs, diffDCs, or LPS-diffDCs at a DC/NK ratio of 1:5. After 24 hours of coculture, cells were harvested for FACS analysis.
Cytotoxic assay
After coculture with DCs for 18 hours, the live NK cells were collected as effectors. Target cells (YAC-1) were incubated with 100 μCi (3.7 MBq) Na 512CrO4 for 1.5 hours at 37°C and then extensively washed. 51Cr-labeled target cells were incubated with effector cells of various effector-to-target (E/T) ratios. Cytotoxicity was measured as 51Cr release following 4 hours of culture at 37°C. The percentage of specific 51Cr release was calculated according to the formula: specific lysis % = [(cpm experimental release – cpm spontaneous release) / (cpm maximal release – cpm spontaneous release)] × 100.
Statistical analysis
Data are shown as mean plus or minus the standard deviation (SD) for separate experiments. Statistical significance was determined by Student t test, with a value of P less than .05 considered as statistically significant.
Results
TLR agonists promote diffDC production of more IL-10 but minimal IL-12p70, without reversing the inability of stimulating T-cell proliferation
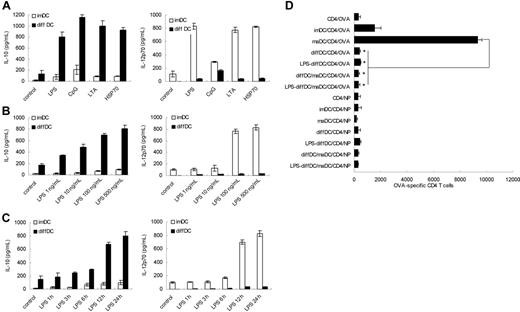
As reported previously, diffDCs secreted significantly higher levels of IL-10 but lower levels of IL-12p70 than imDCs and maDCs, and such regulatory DCs produce significant IL-10 but minimal IL-12p70, ignoring the presence of LPS.10 In light of these results, we hypothesized that production of high IL-10 but little IL-12p70 is the intrinsic characteristic of diffDCs. To investigate whether TLR activation could diminish the Th2 bias, diffDCs and imDCs were stimulated with TLR agonists including LPS, CpG ODN, LTA, or HSP70. As shown in Figure 1A, the bias in the profile of IL-10 and IL-12p70 production was more pronounced following stimulation with every kind of TLR agonist, suggesting that the increased preferential IL-10 production is the universal characteristic of diffDCs in response to inflammatory stimuli. For example, diffDCs were stimulated with a series of concentrations of LPS. LPS induced the production of IL-10 by diffDCs in a dose-dependent manner, and all the concentrations of LPS could not reverse the bias in IL-10 and IL-12p70 production (Figure 1B). imDCs and diffDCs were also stimulated with 500 ng/mL LPS for various times; the suppressed IL-12p70 and increased IL-10 production were consistently observed in diffDCs, as predicted, and increased IL-12p70 production and almost unchanged IL-10 production were observed in imDCs (Figure 1C). These results suggested that the Th2-bias cytokine production is the steady and intrinsic characteristic of diffDCs, and more significant in diffDCs after stimulation with TLR agonists.
diffDCs preferentially produce a high level of IL-10 but a low level of IL-12p70 and inhibit T-cell proliferation, ignoring TLR activation. (A) IL-10 and IL-12p70 production was measured by ELISA following stimulation of imDCs or diffDCs (1 × 106 cells/mL) in 48-well plates with 500 ng/mL LPS, 1 μmol/mL CpG ODN, 5 ng/mL LTA, or 50 ng/mL HSP70 for 24 hours. Unstimulated DCs were used as a control. (B) IL-10 and IL-12p70 production by DCs after stimulation with various concentrations of LPS (1 ng/mL-500 ng/mL) for 24 hours. (C) The concentration of IL-10 and IL-12p70 by DCs following stimulation with 500 ng/mL LPS for various lengths of time. Unstimulated DCs were used as a control. Values in panels A to C are expressed as means plus or minus standard deviation. (D) Purified DO11.10 CD4+ T cells were cocultured with imDCs or maDCs or/and diffDCs treated with or without 500 ng/mL LPS for 24 hours in the presence of OVA323-339 at a ratio of 1:10 (DC/T); LCMV-NP309-328 (NP) was used as an Iab-restricted peptide control. After 5 days, cells were collected and double-stained with anti-CD4–FITC and 7-AAD and counted by FACS. *P < .05.
diffDCs preferentially produce a high level of IL-10 but a low level of IL-12p70 and inhibit T-cell proliferation, ignoring TLR activation. (A) IL-10 and IL-12p70 production was measured by ELISA following stimulation of imDCs or diffDCs (1 × 106 cells/mL) in 48-well plates with 500 ng/mL LPS, 1 μmol/mL CpG ODN, 5 ng/mL LTA, or 50 ng/mL HSP70 for 24 hours. Unstimulated DCs were used as a control. (B) IL-10 and IL-12p70 production by DCs after stimulation with various concentrations of LPS (1 ng/mL-500 ng/mL) for 24 hours. (C) The concentration of IL-10 and IL-12p70 by DCs following stimulation with 500 ng/mL LPS for various lengths of time. Unstimulated DCs were used as a control. Values in panels A to C are expressed as means plus or minus standard deviation. (D) Purified DO11.10 CD4+ T cells were cocultured with imDCs or maDCs or/and diffDCs treated with or without 500 ng/mL LPS for 24 hours in the presence of OVA323-339 at a ratio of 1:10 (DC/T); LCMV-NP309-328 (NP) was used as an Iab-restricted peptide control. After 5 days, cells were collected and double-stained with anti-CD4–FITC and 7-AAD and counted by FACS. *P < .05.
TLR agonists have been shown to activate DCs to produce Th1 cytokine and initiate a Th1 adaptive immune response. Also, it was reported that TLR signaling can reverse the inhibitory function of regulatory T cells.22,23 Therefore, we questioned whether TLR agonists can induce diffDCs to be active in T-cell proliferation or reverse the inhibitory function of diffDCs for CD4+ T-cell proliferation. OVA323-339-specific TCR transgenic CD4+ T cells purified from DO11.10xC57BL/6 F1 mice were used as responders to imDCs or diffDCs that were stimulated with LPS and loaded with OVA323-339 or LCMV-NP309-328 (Iab-restricted peptide antigen control) peptides. After 5 days, viable CD4+ T cells present in T-cell/DC cultures were counted by flow cytometry. As shown in Figure 1D, in spite of the presence of LPS, diffDCs could not enhance antigen-specific T-cell proliferation, but significantly inhibited maDC-induced antigen-specific CD4+ T-cell proliferation. Accordingly, as compared with the phenotype of LPS-induced maDCs, LPS-stimulated diffDCs still expressed lower levels of costimulatory molecules such as CD86 (data not shown). So, diffDCs cannot stimulate but do inhibit antigen-specific CD4+ T-cell proliferation following LPS stimulation. Together with TLR agonist-induced higher IL-10 production in diffDCs, these data indicate TLR agonists cannot reverse the inhibitory function of diffDCs.
The impaired IL-12p70 production in diffDCs is not due to high IL-10 production
IL-10 can inhibit IL-12 secretion by DCs, thus impairing the ability of DCs to generate Th1 responses.24 Since diffDCs express a high level of IL-10 even in the absence of TLR agonists, we examined the role of IL-10 in the impaired IL-12p70 production in diffDCs. As shown in Figure 2A, neutralizing anti–mouse IL-10 antibody failed to increase IL-12p70 production in both LPS-stimulated and unstimulated diffDCs. However, neutralizing anti–mouse IL-10 antibody could increase IL-12p70 production in imDCs after LPS stimulation. diffDCs and imDCs were also prepared from IL-10–deficient mice, and we found that IL-10 deficiency did not affect the production of IL-12p70 in diffDCs with or without LPS stimulation, but promoted IL-12p70 production in imDCs stimulated with 100 ng/mL or 500 ng/mL LPS (Figure 2B). Such data demonstrated that the impaired IL-12p70 production in diffDCs is not due to high IL-10 production.
MAPKs are differentially regulated in diffDCs
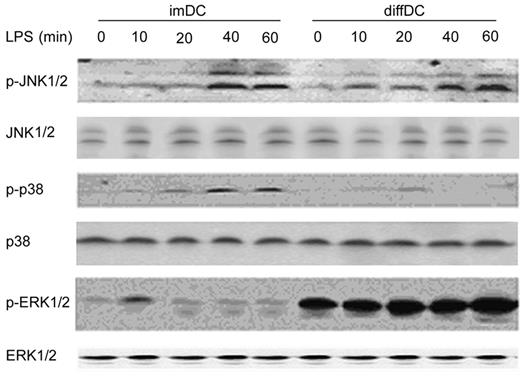
MAPKs, including ERK, JNK, and p38, play different roles in IL-10 and IL-12 production.25-28 Thus we wanted to know whether ERK, JNK, and p38 are differentially regulated in diffDCs and imDCs. As shown in Figure 3, compared with imDCs, diffDCs expressed a higher level of activated ERK1/2 but a lower level of the activated p38; however, JNK was comparably activated in diffDCs and imDCs. LPS stimulation could activate MAPK signal pathways in DCs.29 We compared the activation of the MAPKs in LPS-stimulated diffDCs and imDCs. Similarly to that before LPS treatment, LPS-stimulated diffDCs expressed a higher level of activated ERK1/2 but a lower level of the activated p38. In contrast, LPS-stimulated imDCs exhibited a higher level of activated p38 but a lower level of the activated ERK1/2. JNK was comparably activated by LPS in both diffDCs and imDCs. The tendency in the increased ERK activation and suppressed p38 activation in diffDCs is consistent with that in LPS-induced increased IL-10 but little IL-12p70 in diffDCs, and the reverse tendency is observed in LPS-induced imDCs. The results suggest that the ERK1/2 and p38 pathways are differentially regulated, which might play a role in the differential production of IL-10 and IL-12p70 in diffDCs and imDCs with or without TLR agonist stimulation.
Impaired IL-12p70 production in diffDCs is not due to high IL-10 production. (A) imDCs and diffDCs were pretreated with 10 μg/mL anti–IL-10 antibody or isotype antibody for 30 minutes, treated with 500 ng/mL LPS for 24 hours, and then the level of IL-12p70 in supernatants was determined by ELISA. Untreated DCs were used as a control. *P < .05. (B) imDCs and diffDCs from wild-type or IL-10–/– mice (IL-10–/– imDC and IL-10–/– diffDC) were stimulated with various concentrations of LPS (1 ng/mL-500 ng/mL) for 24 hours. Supernatant was measured using an ELISA kit for IL-12p70. Untreated DCs were used as a control. *P < .05.
Impaired IL-12p70 production in diffDCs is not due to high IL-10 production. (A) imDCs and diffDCs were pretreated with 10 μg/mL anti–IL-10 antibody or isotype antibody for 30 minutes, treated with 500 ng/mL LPS for 24 hours, and then the level of IL-12p70 in supernatants was determined by ELISA. Untreated DCs were used as a control. *P < .05. (B) imDCs and diffDCs from wild-type or IL-10–/– mice (IL-10–/– imDC and IL-10–/– diffDC) were stimulated with various concentrations of LPS (1 ng/mL-500 ng/mL) for 24 hours. Supernatant was measured using an ELISA kit for IL-12p70. Untreated DCs were used as a control. *P < .05.
ERK1/2, JNK1/2 and p38 MAPK pathways are differentially activated in imDCs and diffDCs. imDCs and diffDCs (1 × 106) were treated with 500 ng/mL LPS for 0 to 60 minutes, and were then lysed. The phosphorylation of JNK1/2 (p-JNK1/2), p38 (p-p38), and ERK1/2 (p-ERK1/2) was detected by Western blotting using specific antibodies. Total JNK1/2, p38, and ERK1/2 in each sample were used as the equal loading control. The same blot was used for each different antibody after stripping of the previous antibody. Similar results were obtained in 3 indepedent experiments.
ERK1/2, JNK1/2 and p38 MAPK pathways are differentially activated in imDCs and diffDCs. imDCs and diffDCs (1 × 106) were treated with 500 ng/mL LPS for 0 to 60 minutes, and were then lysed. The phosphorylation of JNK1/2 (p-JNK1/2), p38 (p-p38), and ERK1/2 (p-ERK1/2) was detected by Western blotting using specific antibodies. Total JNK1/2, p38, and ERK1/2 in each sample were used as the equal loading control. The same blot was used for each different antibody after stripping of the previous antibody. Similar results were obtained in 3 indepedent experiments.
Increased ERK1/2 activation is responsible for the preferential expression of IL-10 in diffDCs
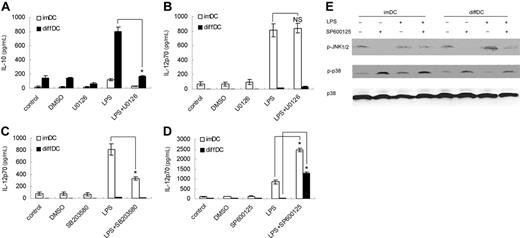
To investigate the role of the ERK1/2 pathway in the differential production of IL-10 and IL-12p70 in diffDCs, diffDCs and imDCs were pretreated with U0126, an ERK1/2 pathway inhibitor. As reported before,27 U0126 pretreatment could inhibit IL-10 but not IL-12p70 production in imDCs with or without LPS stimulation. We found U0126 pretreatment could inhibit IL-10 expression in diffDCs, indicating ERK activation is necessary for diffDCs to produce high IL-10 production, one intrinsic characteristic of diffDCs (Figure 4A). Moreover, the LPS-induced high IL-10 expression in diffDCs was significantly diminished by U0126 pretreatment, suggesting that ERK1/2 activation might play an important role in the up-regulation of IL-10 production in diffDCs induced by LPS. By contrast, IL-12p70 production was not remarkably affected by U0126 pretreatment (Figure 4B).
The suppressed p38 pathway is responsible for the impaired IL-12p70 production in diffDCs
p38 activation is necessary for IL-12 production.28,30 Pretreatment with p38 inhibitor SB203580 significantly blocked IL-12p70 production in imDCs after LPS stimulation (Figure 4C), suggesting the suppressed activation of p38 in diffDCs might contribute to the minimal production of IL-12p70. Interestingly, JNK inhibitor SP600125 not only increased LPS-induced IL-12p70 production in both diffDCs and imDCs, but also reduced the difference in IL-12p70 production between diffDCs and imDCs (Figure 4D). Since JNK was comparably activated in diffDCs and imDCs, the reduced difference of IL-12p70 expression between diffDCs and imDCs could not be accounted for by the inhibition of JNK pathway. Further experiments showed that SP600 125 pretreatment also enhanced p38 activation in diffDCs (Figure 4E), suggesting SP600 125 might affect IL-12p70 expression through reversing the suppression of the p38 pathway in diffDCs, and providing additional evidence that the suppressed p38 pathway is involved in the impaired IL-12p70 production in diffDCs.
Increased ERK activation is required for IL-10 overexpression, and the suppressed p38 pathway is involved in the impaired IL-12p70 production in diffDCs. (A-D) imDCs and diffDCs were pretreated with 10 μmol/mL U0126 (A,B), 10 μmol/mL SB203 580, or 50 μmol/mL SP600 125 for 30 minutes, then stimulated with 500 ng/mL LPS for 24 hours. Equal amounts of DMSO contained in medium were used as negative controls. The levels of IL-10 and IL-12p70 in the supernatants were determined by ELISA. Data were shown as mean plus or minus SD of 3 independent experiments. *P < .05. NS indicates not significant. (E) SP600 125 enhanced p38 activation in imDCs and diffDCs. diffDCs and imDCs were pretreated with 50 μmol/mL SP600 125 for 30 minutes, then stimulated with LPS for 30 minutes. p38 phosphorylation was examined by immunoblotting of cell lysates with anti–phospho-p38 antibody. The membrane was then stripped and total p38 was detected. Similar results were obtained from 3 independent experiments.
Increased ERK activation is required for IL-10 overexpression, and the suppressed p38 pathway is involved in the impaired IL-12p70 production in diffDCs. (A-D) imDCs and diffDCs were pretreated with 10 μmol/mL U0126 (A,B), 10 μmol/mL SB203 580, or 50 μmol/mL SP600 125 for 30 minutes, then stimulated with 500 ng/mL LPS for 24 hours. Equal amounts of DMSO contained in medium were used as negative controls. The levels of IL-10 and IL-12p70 in the supernatants were determined by ELISA. Data were shown as mean plus or minus SD of 3 independent experiments. *P < .05. NS indicates not significant. (E) SP600 125 enhanced p38 activation in imDCs and diffDCs. diffDCs and imDCs were pretreated with 50 μmol/mL SP600 125 for 30 minutes, then stimulated with LPS for 30 minutes. p38 phosphorylation was examined by immunoblotting of cell lysates with anti–phospho-p38 antibody. The membrane was then stripped and total p38 was detected. Similar results were obtained from 3 independent experiments.
Enhanced NF-κB activation is not involved in preferential IL-10 production by diffDCs
The NF-κB pathway plays important roles in proinflammatory cytokine production.31,32 Thus we tested the activation of the NF-κB pathway in diffDCs and imDCs and wanted to know whether the NF-κB pathway is involved in the unique cytokine profile of diffDCs. As shown in Figure 5A, NF-κBp65 was comparably expressed in diffDCs and imDCs, but the phosphorylation of NF-κBp65 in diffDCs was enhanced, more than that in imDCs with or without LPS stimulation. The degradation of IκB-α and the nuclear translocation of NF-κBp65 following LPS stimulation were also detected. In imDCs, LPS induced nuclear translocation of the NF-κBp65 subunit within 20 minutes. In contrast, there was already abundant NF-κBp65 subunit in the nuclear translocation of diffDCs before LPS stimulation. Consistently, IκB-α was gradually degraded, induced by LPS in imDCs, but could not be detected in diffDCs even before LPS stimulation (Figure 5B). To further study the role of NF-κB activation in the regulation of IL-10 and IL-12p70 production, diffDCs and imDCs were stimulated with LPS in the presence of 100 μmol/mL PDTC. As shown in Figure 5C, PDTC pretreatment could not inhibit preferential IL-10 production in diffDCs, and also could not affect the LPS-induced increase in IL-10 and impaired IL-12p70 secretion in diffDCs. However, PDTC pretreatment efficiently inhibited the LPS-induced IL-12p70 production in imDCs. So, NF-κB activation, although enhanced in diffDCs, may not be responsible for the Th2-bias cytokine production in diffDCs. The biologic significance of NF-κB activation in diffDCs needs to be further addressed in the future.
NF-κB pathway activation is enhanced in diffDCs but is not responsible for the unique cytokine profile of diffDCs. (A) imDCs and diffDCs were cultured in medium alone as a control (0) or were stimulated with 500 ng/mL LPS for the indicated times. Whole-cell lysates were then electrophoresed and probed with phospho–NF-κBp65, followed by an appropriate secondary antibody. (B) Degradation of IκB-α and nuclear translocation of NF-κB was enhanced in diffDCs. Cytoplasmic or nuclear extracts from DCs cultured in medium alone or stimulated with 500 ng/mL LPS for 10, 20, 40, or 60 minutes were prepared, blotted, and probed with IκB- and NF-κB–specific antibodies, respectively. (C) NF-κB activation was not required for the IL-10 overexpression and impaired IL-12p70 secretion in diffDCs. DCs were pretreated with 100 μmol/mL PDTC, an inhibitor of NF-κB activation, for 30 minutes, then stimulated with LPS for 24 hours. Levels of IL-10 and IL-12p70 in supernatants were determined by ELISA. *P < .05. NS indicates not significant.
NF-κB pathway activation is enhanced in diffDCs but is not responsible for the unique cytokine profile of diffDCs. (A) imDCs and diffDCs were cultured in medium alone as a control (0) or were stimulated with 500 ng/mL LPS for the indicated times. Whole-cell lysates were then electrophoresed and probed with phospho–NF-κBp65, followed by an appropriate secondary antibody. (B) Degradation of IκB-α and nuclear translocation of NF-κB was enhanced in diffDCs. Cytoplasmic or nuclear extracts from DCs cultured in medium alone or stimulated with 500 ng/mL LPS for 10, 20, 40, or 60 minutes were prepared, blotted, and probed with IκB- and NF-κB–specific antibodies, respectively. (C) NF-κB activation was not required for the IL-10 overexpression and impaired IL-12p70 secretion in diffDCs. DCs were pretreated with 100 μmol/mL PDTC, an inhibitor of NF-κB activation, for 30 minutes, then stimulated with LPS for 24 hours. Levels of IL-10 and IL-12p70 in supernatants were determined by ELISA. *P < .05. NS indicates not significant.
diffDC-derived IL-10 contributes to activation of NK cells induced by diffDCs
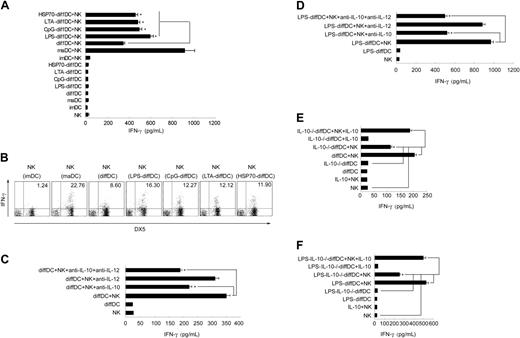
Lastly, we wanted to know the role of IL-10 overexpression in the regulation of immune response by diffDCs. Our previous study demonstrated that diffDC-derived IL-10 is not involved in the diffDC inhibition of T-cell proliferation.10 By screening the biologic significance of diffDCs in immune regulation, we interestingly found that diffDCs could activate NK cells, and that diffDC-derived IL-10 is involved in this process. We examined diffDC-NK interaction by measuring IFN-γ production in supernatant of cocultures. The induction of IFN-γ by diffDCs was dramatically higher than that by imDCs but lower than that by maDCs. Stimulated with TLR agonists including LPS, CpG ODN, LTA, or HSP70, diffDCs induced more IFN-γ production than that induced by unstimulated diffDCs (Figure 6A). To verify that IFN-γ was produced by NK cells rather than diffDCs, the presence of IFN-γ–positive NK cells was examined by intracellular staining of DX5+ cells after 18 hours of coculture in the presence of 10 μg/mL Brefeldin A. As shown in Figure 6B, 22.76%, 8.60%, 16.30%, 12.27%, 12.12%, or 11.90% of NK cells produced IFN-γ after coculture with maDCs, diffDCs, LPS-diffDCs, CpG-diffDCs, LTA-diffDCs, and HSP70-diffDCs, respectively. In contrast, IFN-γ–positive diffDCs or TLR agonist-diffDCs were not detected in the coculture (data not shown). However, imDCs did not activate NK cells in coculture to produce IFN-γ or up-regulate the expression of NK-cell activation markers. As reported previously by us, diffDCs secrete considerable amounts of IL-10 but low amounts of IL-12p70.10 Using specific neutralizing mAbs, we then tested the role of these cytokines in the activation of NK cells induced by diffDCs or LPS-diffDCs. Surprisingly, anti–IL-10 antibody inhibited IFN-γ production by NK cells induced by diffDCs with or without LPS stimulation. In contrast, IL-12 blockade had little effect on NK-cell IFN-γ production induced by DCs (Figure 6C-D). To further test the role of diffDC-derived IL-10 in such NK-cell activation, we generated IL-10–/–diffDCs or LPS-IL-10–/–diffDCs and then tested their capability to activate NK cells. Supernatants from diffDC/NK cocultures were collected 24 hours later, and IFN-γ production was measured. Compared with diffDCs or LPS-diffDCs, the function to induce IFN-γ production by NK cells was obviously impaired in IL-10–/–diffDCs or LPS-IL-10–/–diffDCs and was recovered by exogenous IL-10 (Figure 6E-F). However, splenic resting NK cells that were incubated only with exogenous IL-10 at the indicated concentration did not increase IFN-γ secretion, suggesting that IL-10 alone is not sufficient to activate NK cells. Together, these results suggest that similar to maDC-mediated activation of NK cells, diffDCs with or without LPS stimulation could activate NK cells to produce IFN-γ, and diffDC-derived IL-10 contributes to NK-cell activation by diffDCs.
IL-10 is involved in diffDC-induced activation of NK cells. (A) DCs or TLR agonist-DCs (1 × 105) were incubated with purified splenic resting NK cells. After 24 hours of coculture, the supernatants were collected and analyzed by ELISA for IFN-γ production. (B) Intracellular staining for IFN-γ expression in splenic NK cells cocultured with various DCs in the presence of 10 μg/mL Brefeldin A for 18 hours. (C-F) Before being incubated with NK cells, 1.0 × 105 diffDCs or LPS-diffDCs were pretreated with anti–IL-10, anti–IL-12, or both anti–IL-10 and anti–IL-12 neutralizing mAbs (5 μg/mL) for 1 hour, then were cocultured with splenic resting NK cells. Similar to diffDC-NK coculture, 1.0 × 105 IL-10–/– diffDCs (with or without 500 ng/mL LPS stimulation) were cocultured with splenic resting NK cells. In some experiments, the exogenous 300 pg/mL (left) or 700 pg/mL (right) IL-10 was added into IL-10–/– diffDC-NK-cell or IL-10–/– LPS-diffDC-NK-cell coculture, respectively. After 24 hours of coculture, the supernatants were collected to measure IFN-γ production by ELISA. The data shown here were obtained in mean of triplicates. The diffDC/NK ratio in all these experiments was 1:5. *P < .05.
IL-10 is involved in diffDC-induced activation of NK cells. (A) DCs or TLR agonist-DCs (1 × 105) were incubated with purified splenic resting NK cells. After 24 hours of coculture, the supernatants were collected and analyzed by ELISA for IFN-γ production. (B) Intracellular staining for IFN-γ expression in splenic NK cells cocultured with various DCs in the presence of 10 μg/mL Brefeldin A for 18 hours. (C-F) Before being incubated with NK cells, 1.0 × 105 diffDCs or LPS-diffDCs were pretreated with anti–IL-10, anti–IL-12, or both anti–IL-10 and anti–IL-12 neutralizing mAbs (5 μg/mL) for 1 hour, then were cocultured with splenic resting NK cells. Similar to diffDC-NK coculture, 1.0 × 105 IL-10–/– diffDCs (with or without 500 ng/mL LPS stimulation) were cocultured with splenic resting NK cells. In some experiments, the exogenous 300 pg/mL (left) or 700 pg/mL (right) IL-10 was added into IL-10–/– diffDC-NK-cell or IL-10–/– LPS-diffDC-NK-cell coculture, respectively. After 24 hours of coculture, the supernatants were collected to measure IFN-γ production by ELISA. The data shown here were obtained in mean of triplicates. The diffDC/NK ratio in all these experiments was 1:5. *P < .05.
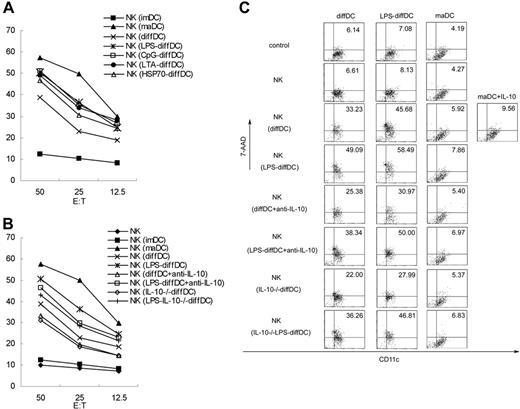
diffDC-activated NK cells in turn acquire the ability to kill diffDCs, partially though IL-10
To further investigate whether diffDCs are able to enhance NK-cell cytolytic activity, we cocultured diffDCs with splenic resting NK cells at a 1:5 ratio for 18 hours, and the ability of NK cells to kill target cells (YAC-1) was then measured. As shown in Figure 7A, coculture with diffDCs enhanced NK-cell cytotoxicity, whereas NK cells cocultured with diffDCs stimulated with TLR agonists had higher cytotoxicity against YAC-1 cells. Additionally, we also observed that coculture with maDCs could enhance NK-cell cytotoxicity against target cells whereas coculture with imDCs had no obvious effect on NK-cell cytolytic activity. Furthermore, we found that anti–IL-10 antibody could partially inhibit the cytotoxicity to YAC-1 cells of NK cells induced by diffDCs with or without LPS stimulation. To further confirm the involvement of diffDC-derived IL-10 in the activation of NK cells, IL-10–/–diffDCs or LPS-IL-10–/–diffDCs were generated and then used to coculture NK cells. We found that IL-10 deficiency did decrease the cytotoxicity of such NK cells (Figure 7B). It has been proposed that DC-activated NK cells can kill autologous imDCs efficiently in vitro.18 We then asked whether diffDC-activated splenic NK cells acquired the ability to kill DCs. Indeed, a large fraction of freshly isolated diffDCs was lysed by diffDC-activated splenic NK cells. Moreover, an even higher percentage of LPS-stimulated diffDCs was lysed by diffDC-activated NK cells. However, maDCs were resistant to lysis of diffDC-activated NK cells (Figure 7C). To determine whether diffDC-derived IL-10 is responsible for the lysis, we tested the effects of anti–IL-10 antibody in this system. IL-10 blockade could partially inhibit the cytotoxic ability of diffDC- or LPS-diffDC–activated NK cells to the freshly isolated diffDCs and LPS-diffDCs. The IL-10–/–diffDCs or LPS-IL-10–/– diffDCs impaired such function, similar to those IL-10 antibody–neutralized diffDCs or LPS-diffDCs. However, even with the addition of exogenous IL-10, maDCs were still resistant to NK-mediated cytotoxicity, thus excluding the possibility that there is an autocrine effect of IL-10 on diffDCs or LPS-diffDCs in the susceptibility for NK-induced cytotoxicity. Our results indicated that, partially through IL-10, diffDCs with or without TLR agonist stimulation could activate splenic resting NK cells, which, in turn, acquired the ability to kill diffDCs.
IL-10 is partially required for the stimulation of NK cytotoxicity by diffDCs. (A-B) Splenic NK cells (5 × 105) were cocultured with diffDCs, LPS-diffDCs, CpG-diffDCs, LTA-diffDCs, HSP70-diffDCs, maDCs, or imDCs for 18 hours. In some experiments, before being incubated with NK cells, 1.0 × 105 diffDCs or LPS-diffDCs were pretreated with anti–IL-10 antibody (5 μg/mL) for 1 hour, then cocultured with splenic resting NK cells. Similar to diffDC-NK coculture, 1.0 × 105 IL-10–/– diffDCs (with or without 500 ng/mL LPS stimulation) were cocultured with NK cells. Then the cytotoxicity of NK cells against the target cells (YAC-1) was tested in a standard 4-hour 51Cr release assay at various effector-to-target ratios. The data shown here were obtained in mean of triplicates. The DC/NK ratio in these experiments was 1:5. (C) FACS analysis of NK killing of DCs. After 24 hours of coculture, cells were harvested and then stained with specific immunofluorescence-conjugated anti-CD11c mAb and 7-AAD. In the maDC plus IL-10 group, the exogenous 700 pg/mL IL-10 was added into maDC-NK-cell coculture. The dot plots were derived from gated events with forward- and side-light scatter characteristics of DCs.
IL-10 is partially required for the stimulation of NK cytotoxicity by diffDCs. (A-B) Splenic NK cells (5 × 105) were cocultured with diffDCs, LPS-diffDCs, CpG-diffDCs, LTA-diffDCs, HSP70-diffDCs, maDCs, or imDCs for 18 hours. In some experiments, before being incubated with NK cells, 1.0 × 105 diffDCs or LPS-diffDCs were pretreated with anti–IL-10 antibody (5 μg/mL) for 1 hour, then cocultured with splenic resting NK cells. Similar to diffDC-NK coculture, 1.0 × 105 IL-10–/– diffDCs (with or without 500 ng/mL LPS stimulation) were cocultured with NK cells. Then the cytotoxicity of NK cells against the target cells (YAC-1) was tested in a standard 4-hour 51Cr release assay at various effector-to-target ratios. The data shown here were obtained in mean of triplicates. The DC/NK ratio in these experiments was 1:5. (C) FACS analysis of NK killing of DCs. After 24 hours of coculture, cells were harvested and then stained with specific immunofluorescence-conjugated anti-CD11c mAb and 7-AAD. In the maDC plus IL-10 group, the exogenous 700 pg/mL IL-10 was added into maDC-NK-cell coculture. The dot plots were derived from gated events with forward- and side-light scatter characteristics of DCs.
Discussion
In our previous study, we identified a new regulatory subset of DCs in vivo, namely diffDCs, which inhibit rather than activate antigen-specific T-cell proliferation.10 diffDCs display a Th2-biased cytokine profile by secreting more IL-10 but less IL-12p70. However, whether stimulation by invading pathogens can switch the unique cytokine profile of diffDCs and reverse their regulatory role in innate and adaptive immunity remains unknown. Here, we further demonstrate that overactivation of ERK and suppression of p38 MAPK pathways contribute to the unique cytokine profile of diffDCs in the steady state, which cannot be reversed but becomes more pronounced after stimulation with TLR agonists. Accordingly, the inhibition of T-cell proliferation by diffDCs cannot be reversed by TLR agonists. Interestingly, we also observed that, partially by overexpressed IL-10, diffDCs could induce NK cells to secrete IFN-γ and the diffDC-activated NK cells could in turn kill diffDCs. Moreover, TLR agonist stimulation further enhanced the activating effects of diffDCs on NK cells. In this way, diffDCs appear to exert their unique and powerful regulation of immune and inflammatory responses.
TLRs are abundantly expressed on professional APCs such as macrophages and DCs, and served as an important link between the innate and adaptive immune responses.33 TLR-mediated recognition of microbial components by imDCs induces dynamic and coordinated reprogramming of gene expression, surface phenotype, and cellular function.34 This process is known as DC maturation, by which TLRs control the adaptive immune responses. It has been shown that there are several mechanisms that control T-cell responses which cannot be regulated only by CD4+CD25+ suppressor or Treg cells, but also by the APCs. In particular, TLR agonists can activate or reverse the inhibitory function of Treg cells in a DC-dependent or -independent manner.22,23 Whether such TLR signals directly regulate the suppressive function of diffDCs remains unknown. So, we observed the effects of LPS on the function and cytokine production profile of diffDCs. However, our data showed that LPS treatment could not reverse the inhibitory function of diffDCs on T-cell proliferation; furthermore, LPS and other TLR agonist treatment even amplified the bias between IL-10 and IL-12p70 production. Interestingly, IL-10 is involved in the activation of splenic NK cells mediated by diffDCs especially after LPS stimulation, which in turn lyse the surrounding diffDCs. Our findings suggest that diffDCs may retain their negative regulatory role during infection and further promote the appropriate downstream immune responses for defense against pathogens.
It is believed that DCs, which present tissue- or organ-specific peptides derived from necrotic cells under inflammatory conditions, can prime autoreactive T cells, resulting in autoimmunity. An alternative mechanism has been suggested that TLR agonists such as LPS and CpG ODN induce mouse DCs to secrete cytokines that render CD4+ effector cells refractory to Treg cell–mediated suppression or convert tolerogenic DCs presenting self-Ags into autoimmunogenic DCs.22,35-37 However, if TLR agonists alone were sufficient to break self-tolerance, the activation of autoimmunity should be easily followed after infections with TLR-bearing pathogens or use of some vaccine adjuvants. But in fact, infections are familiar while organ-specific autoimmune diseases are rare. Our results that LPS-stimulated diffDCs can secrete more IL-10 and significantly inhibit maDC-induced antigen-specific CD4+ T-cell proliferation support the notion that diffDCs may play certain roles in prevention of autoimmune disorders after infection.
Although many studies have investigated DC-NK interaction, the underlying mechanisms of DC-NK crosstalks are not fully understood.38 Many cytokines have been reported to be crucial in DC stimulation of NK activation in vitro, including IL-12, IL-18, IL-2, and type I IFN.18,39 Of note, IL-12 was generally accepted as a key NK activation cytokine derived from maDCs. Here we found that neutralization of IL-10, but not IL-12, reduced IFN-γ production in diffDC-NK coculture. Moreover, the role of IL-10 in diffDC-induced NK activation was further confirmed by using IL-10–/–diffDCs. It has also been reported that IL-10 can induce NK-cell activation.40-42 We also analyzed the effects of splenic NK cells on diffDCs. These splenic NK cells, once activated by diffDCs, partially through IL-10, acquired the ability to lyse surrounding diffDCs but not maDCs. NK-mediated lysis of diffDCs might contribute to sustain the homeostasis of diffDCs in steady state. In the case of infection, diffDCs stimulated by pathogens (mimicked by LPS or other TLR agonist-diffDCs) could remarkably induce NK-cell activation and might provide an early source of IFN-γ that is necessary for Th1 polarization. Thus, in turn, killing of diffDCs stimulated by pathogens might avoid excessive IFN-γ–involved activation-induced cell death (AICD) of T lymphocytes. Although diffDC-derived IL-10 plays an important role in the activation of NK cells, it may be only one boosting factor for NK cells. Furthermore, despite the addition of exogenous IL-10, maDCs are still resistant to NK cell–mediated cytotoxicity as compared with diffDCs or LPS-diffDCs, suggesting that there must be other mediators involved in this process, and more precise mechanisms underlying this phenomenon need to be further investigated.
DCs of different subsets or undergoing distinct functional states recognize PAMPs present in microbes through TLRs and are thereby activated to produce IL-10 and IL-12. Cytokines secreted by DCs play an important role in directing T-cell responses. Kinetic study of IL-12 and IL-10 production by DCs clearly shows that IL-12 is a key cytokine in innate responses that can drive Th1 polarization, while IL-10–producing DCs prime Th2 response.1,43 IL-10 is generally considered to limit immune and inflammatory responses and plays an important role in maintaining the balance between Th1 and Th2 responses.44,45 In addition, IL-10–producing DCs have been described to be able to induce regulatory T cells.46 Thus, the Th2-biased cytokine profile characterized by high IL-10 production but minimal IL-12p70 production in both resting and LPS-stimulated diffDCs might be important for their function. We sought to illuminate the signaling events underlying the unique cytokine profile of diffDCs. Some previous studies suggested that IL-10 production could inhibit IL-12 production.24,47 However, neither neutralizing anti–IL-10 antibody nor endogenous IL-10 deficiency could increase IL-12p70 production in diffDCs, indicating that the minimal IL-12p70 production in diffDCs is not due to the presence of a large amount of IL-10. It has been shown that stimulation with some TLR agonist in presence of anti–IL-10 antibody could revert tumor-induced DC paralysis and trigger de novo IL-12 production.48 But in our experiments, LPS plus anti–IL-10 antibody treatment or at a setting of IL-10 deficiency could not enhance IL-12p70 secretion in diffDCs. A possible explanation was that diffDCs might have an intrinsic characteristic with potential to secrete a high level of IL-10, which subsequently facilitated proper control of immune response during or after infection.
ERK1/2 is necessary for IL-10 production but might inhibit IL-12 production.27,49,50 Therefore, it is of interest to determine whether a high level of IL-10 but an almost undectectable level of IL-12p70 production by diffDCs involves heightened phosphorylation of ERK. We investigated the activation of ERK in imDCs and diffDCs. As anticipated, ERK activation is enhanced in diffDCs as compared with imDCs. Furthermore, LPS-stimulated diffDCs display a higher level of ERK phosphorylation, in line with the higher IL-10 production. However, ERK activation augmented IL-10 production but did not inhibit IL-12p70 production in diffDCs since ERK inhibitor suppressed IL-10 production but failed to increase IL-12p70 production in diffDCs. This suggests that the up-regulated IL-10 production might be due to enhanced activation of ERK1/2 in diffDCs.
In summary, our present study demonstrates that regulatory DCs (diffDCs) derived from maDCs under stroma can still function as a negative regulator of immune response even after activated by TLR agonists. The Th2-biased cytokine production profile in diffDCs is correlated with enhanced ERK1/2 activation and suppressed p38 activation, and the profile cannot be reversed by TLR activation. In addition to the inhibitory effects of diffDCs on T cells, diffDCs interacting reciprocally with NK cells might provide a new way for their important regulation of immune responses.
Prepublished online as Blood First Edition Paper, June 15, 2006; DOI 10.1182/blood-2006-03-005595.
Supported by grants from the National Key Basic Research Program of China (2001CB510002) and the National Natural Science Foundation of China (nos. 30490240, 30130170, 30121002).
C.Q. and X.J. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We sincerely appreciate Ms Xianwei Ma and Ms Rui Zhang for their excellent technical assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal