Abstract
Hyperhomocysteinemia is a risk factor for thrombosis, but the mechanisms are not well defined. We tested the hypothesis that hyperhomocysteinemia accelerates arterial thrombosis in mice. Mice heterozygous for a targeted disruption of the cystathionine β-synthase gene (Cbs+/–) and wild-type littermates (Cbs+/+) were fed either a control diet or a high methionine/low folate (HM/LF) diet for 6 to 8 months to produce graded hyperhomocysteinemia. The time to occlusion of the carotid artery after photochemical injury was shortened by more than 50% in Cbs+/+ or Cbs+/– mice fed the HM/LF diet (P < .001 versus control diet). Carotid artery thrombosis was not accelerated in mice deficient in endothelial nitric oxide synthase (Nos3), which suggests that decreased endothelium-derived nitric oxide is not a sufficient mechanism for enhancement of thrombosis. Cbs+/+ and Cbs+/– mice fed the HM/LF diet had elevated levels of reactive oxygen species in the carotid artery, increased aortic expression of the NADPH oxidase catalytic subunit, Nox4, and decreased activation of anticoagulant protein C in the aorta (P < .05 versus control diet). We conclude that hyperhomocysteinemia enhances susceptibility to arterial thrombosis through a mechanism that is not caused by loss of endothelium-derived nitric oxide but may involve oxidative stress and impairment of the protein C anticoagulant pathway.
Introduction
Hyperhomocysteinemia is an independent risk factor for cardiovascular disease, ischemic stroke, and venous thromboembolism.1 The strongest evidence that hyperhomocysteinemia produces a prothrombotic state is derived from clinical observations in patients with cystathionine β-synthase (CBS) deficiency,2 who often have severe elevations of plasma total homocysteine (tHcy)3 to levels more than 100 μM. If untreated, about 50% of homozygous CBS-deficient patients suffer from arterial or venous thromboembolic events before the age of 30.2 Treatment with homocysteine-lowering therapy (high-dose pyridoxine, betaine, and restriction of dietary methionine) leads to a dramatic decrease in thromboembolic events.4 An increased risk of arterial and venous thrombosis also has been demonstrated in subjects with moderate hyperhomocysteinemia (plasma tHcy of 10-50 μM),5-7 although it is not yet known whether homocysteine-lowering therapy is beneficial for the primary or secondary prevention of thrombotic events in such subjects.8,9
Despite the strong association between hyperhomocysteinemia and thrombotic risk, the mechanisms responsible for the prothrombotic state of hyperhomocysteinemia are still poorly understood.10 Many potential mechanisms have been proposed, including endothelial dysfunction due to decreased bioavailability of endothelium-derived nitric oxide (NO), enhanced platelet activation, oxidative stress leading to up-regulation of tissue factor, and down-regulation of the protein C anticoagulant pathway.10-12 It is uncertain whether these mechanisms contribute to thrombosis in vivo, however, and no studies have been performed to determine whether susceptibility to experimental thrombosis is altered in hyperhomocysteinemic animals.
In the present study, we tested the hypothesis that hyperhomocysteinemia accelerates carotid artery thrombosis induced by photochemical injury in mice. To produce hyperhomocysteinemia over a range of pathophysiologically relevant concentrations of plasma tHcy, we used a high methionine/low folate (HM/LF) diet with both wild-type and heterozygous cystathionine β-synthase (Cbs)–deficient mice.13,14 To determine whether decreased production of endothelium-derived NO causes accelerated thrombosis, we also examined susceptibility to carotid artery thrombosis in mice deficient in endothelial NO synthase (Nos3). Additional experiments were performed to address the effects of hyperhomocysteinemia on platelet activation, vascular oxidative stress, and the protein C anticoagulant system. Our findings suggest that hyperhomocysteinemia accelerates thrombosis through a mechanism that is not mediated by loss of endothelium-derived NO or abnormal platelet activation but may involve oxidative stress and decreased protein C activation.
Materials and methods
Mice and experimental diets
Animal protocols were approved by the University of Iowa and Veterans Affairs Animal Care and Use Committees. Mice heterozygous for disruption of the Cbs gene13 were crossbred to C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) for at least 9 generations to generate heterozygous (Cbs+/–) and wild-type (Cbs+/+) littermates. Genotyping was performed by polymerase chain reaction using a common forward primer (5′-GGTCTGGAATTCACTATGTAGC-3′) with reverse primers specific for the wild-type (5′-AAGAGCCCAGCAGAATGAACA-3′) or targeted (5′-GAGGTCGACGGTATCGATA-3′) Cbs alleles. At the time of weaning (3-4 weeks of age), mice were fed either a control diet (LM485, Harlan Teklad, Madison, WI), which contains 6.7 mg/kg folic acid and 4.0 g/kg l-methionine, or an HM/LF diet (TD00205, Harlan Teklad) that contains 0.2 mg/kg folic acid and 8.2 g/kg l-methionine.15 Mice were maintained on the control or HM/LF diets until they were studied at 6 to 8 months of age. C57BL/6 mice homozygous for disruption of the endothelial NO synthase (Nos3) gene were purchased from the Jackson Laboratory, fed the control diet, and studied at 6 to 8 months of age.
Carotid artery thrombosis
Carotid artery thrombosis was induced by photochemical injury as described previously.16 Mice were anesthetized with sodium pentobarbital (70-90 mg/kg intraperitoneally) and ventilated mechanically with room air and supplemental oxygen. The left femoral vein was cannulated for the administration of rose bengal. The right common carotid artery was dissected free and carotid artery blood flow was measured with a 0.5 PSB Doppler flow probe (Transonic Systems, Ithaca, NY) and digital recording system (Gould Ponemah Physiology Platform version 3.33; Data Sciences International, Saint Paul, MN). To induce endothelial injury, the right common carotid artery was transilluminated continuously with a 1.5-mV, 540-nm green laser (Melles Griot, Carlsbad, CA) from a distance of 6 cm, and rose bengal (50 mg/kg) was injected via a femoral vein catheter. Blood flow was monitored continuously for 90 minutes or until stable occlusion occurred, at which time the experiment was terminated. First occlusion was defined as the time at which blood flow first decreased to zero for 10 seconds or longer, and stable occlusion was defined as the time at which blood flow remained absent for 10 minutes or longer.
Plasma tHcy, methionine, and folate
Blood was collected by cardiac puncture into EDTA (final concentration 5 mM) and plasma was flash frozen. Plasma tHcy, defined as the total concentration of homocysteine after quantitative reductive cleavage of all disulfide bonds,3 was measured by high-performance liquid chromatography (HPLC) using ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulphonate (SBDF) fluorescence detection.17 Plasma methionine was measured by HPLC coupled to fluorescence detection after precolumn derivatization using o-phthaldialdehyde as described previously.18 Plasma folate was measured with the Quantaphase II folate radioimmunoassay (Bio-Rad Laboratories, Hercules, CA).
Detection of reactive oxygen species
The oxidative fluorescent dye, dihydroethidium (DHE; Invitrogen, Carlsbad, CA), was used to detect reactive oxygen species (ROSs) in frozen sections of common carotid artery by laser scanning confocal microscopy as described previously.19 Control sections were preincubated for 30 minutes with 250 U/mL polyethylene glycol-superoxide dismutase (PEG-SOD; Sigma-Aldrich Company, St Louis, MO) before incubation with DHE. Fluorescent images were analyzed with Scion Image software (Scion, Frederick, MD). Data are reported as the percentage of surface area of carotid sections within the upper 20% of fluorescence intensity.
Platelet activation
Washed platelets were isolated as described previously,20,21 and resuspended in Tyrode buffer (134 mM NaCl, 2.9 mM KCl, 2.9 mM CaCl2, 0.34 mM Na2HPO4, 12 mM NaHCO3, 20 mM HEPES, 1.0 mM MgCl2, 5.0 mM glucose, 0.05% [wt/vol] fatty acid-free BSA, pH 7.35). To assess platelet activation, 2-color flow cytometry was performed after activation of washed platelets with bovine thrombin (0.5 U/mL; Haematological Technologies, Essex Junction, VT), convulxin (250 ng/mL; Centerchem, Norwalk, CT), or convulxin plus thrombin, for 5 minutes at 37°C without stirring. Platelets were then incubated for 10 minutes at 23°C with FITC-conjugated anti–murine CD62P (BD PharMingen, San Diego, CA), FITC-conjugated annexin V (BD PharMingen), or FITC-conjugated sheep anti–human fibrinogen antibody (Novus Biologicals, Littleton, CO) and PE-conjugated JON/A (Emfret Analytics, Würzburg, Germany). Platelets were then fixed in 1% paraformaldehyde, diluted, and analyzed on a Becton Dickinson (San Diego, CA) FACScan flow cytometer as described previously.20,21 Coated platelets were defined as the population of activated platelets with high surface levels of fibrinogen and low PE-JON/A binding.21
Platelet procoagulant activity was measured in a prothrombinase assay as described previously.21 Briefly, washed platelets were either left unstimulated or stimulated with thrombin (0.5 U/mL) or convulxin (200 ng/mL) plus thrombin. After 5 minutes, bovine factor Xa (3.0 nM) and bovine factor Va (6.0 M) were added, followed 1 minute later by bovine prothrombin (4.0 μM). The concentration of CaCl2 in the final reaction mixture was 2.0 mM. After 3 minutes, the rate of thrombin generation was determined by subsampling the reaction mixture into 0.05 M Tris-HCl, 120 mM NaCl, 2.0 mM EDTA, 0.05% BSA, pH 7.5. The thrombin generated was measured using Chromozym TH (1.9 mM; Roche Diagnostics, Indianapolis, IN) in a Spectramax 190 microplate spectrophotometer (Molecular Devices, Sunnyvale, CA).
Protein C activation
Activation of endogenous protein C was measured in response to intravenous injection of thrombin. Mice were anesthetized by inhalation of 75% CO2/25% O2, and 50 μL of either saline or human α-thrombin thrombin (80 U/kg; Haematological Technologies) was administered by retro-orbital injection. After 10 minutes, blood was collected by cardiac puncture into a one-tenth volume of 0.1 M sodium citrate containing 0.5 M benzamidine. The concentration of activated protein C (APC) in citrate/benzamidine-treated plasma was measured using an enzyme-capture enzyme-linked immunosorbent assay (ELISA) described by Li et al.22 APC was captured using monoclonal antibody (mAb) 1587 (a generous gift from Dr Charles Esmon, Oklahoma Medical Research Foundation, Oklahoma City) and APC activity determined spectrophotometrically after 24 hours of incubation with 1.0 mM Spectrozyme PCa (American Diagnostica, Stamford, CT). The assay was standardized with murine APC (Haematological Technologies).
Activation of exogenous protein C by thrombin was measured in freshly isolated rings of proximal aorta (1.0 mm in length) by using a 2-stage assay as described previously.23 Reference curves were generated using rabbit lung thrombomodulin (American Diagnostica). One unit of activity was defined as the amount of APC generated in the presence of 1.0 nM rabbit thrombomodulin.
Real-time PCR
Levels of mRNA for thrombomodulin, the endothelial protein C receptor (EPCR), tissue factor, GAPDH, and the NADPH oxidase subunits Nox1, Nox2, and Nox4 were measured by quantitative real-time polymerase chain reaction (PCR) as described previously.24 Total RNA was isolated from aorta or lung using TRIzol reagent (Invitrogen, Carlsbad, CA) and treated with DNAse I to remove contaminating genomic DNA. RNA (1.5 μg from lung or 0.325 μg from aorta) was then reverse transcribed using TaqMan reverse transcriptase and random hexamer primers as described previously. PCR primers and 6-carboxy fluorescein-labeled probes for GAPDH (Mm99999915_g1), thrombomodulin (Mm00437014_s1), EPCR (Mm00440992_m1), tissue factor (Mm004438853_m1), Nox1 (Mm00549170_m1), Nox2 (Mm00432775_m1), and Nox4 (Mm00479246_m1) were purchased from Applied Biosystems (Foster City, CA). Reverse transcribed cDNA was incubated with TaqMan Universal PCR mix (Applied Biosystems) and PCR primers and probes at 50°C for 2 minutes and then at 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute using the Applied Biosystems 7700 sequence detection system.
Amplicon-specific standard curves generated by serial dilution of cloned cDNA were used to quantify the amount of thrombomodulin, EPCR, and GAPDH cDNA in each sample. Data were analyzed using Sequence Detection software version 1.6.3 (Applied Biosystems) and expressed as a ratio to levels of GAPDH mRNA. For quantitative analysis of mRNA levels for tissue factor, Nox1, Nox2, and Nox4, the comparative threshold cycle (ΔΔCT) method25 was used, with values normalized to GAPDH and expressed relative to levels in Cbs+/+ mice fed the control diet. Validation experiments were performed to confirm equal amplification efficiency for all primers sets.
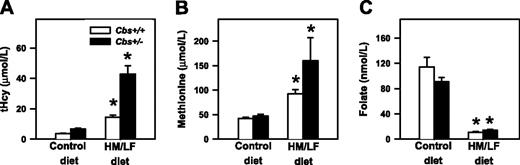
Plasma tHcy, methionine, and folate. Plasma levels of (A) total homocysteine (tHcy), (B) methionine, and (C) folate in Cbs+/+ and Cbs+/– mice fed either control diet or the HM/LF diet (n = 18-23 in each group). Values are mean ± SEM. *P < .05 compared to mice of same genotype fed the control diet.
Plasma tHcy, methionine, and folate. Plasma levels of (A) total homocysteine (tHcy), (B) methionine, and (C) folate in Cbs+/+ and Cbs+/– mice fed either control diet or the HM/LF diet (n = 18-23 in each group). Values are mean ± SEM. *P < .05 compared to mice of same genotype fed the control diet.
Statistical analysis
The 2-way analysis of variance (ANOVA) with the Tukey test for multiple comparisons was used to analyze the effects of Cbs genotype and diet. The 2-tailed t test was used to compare occlusion times in Nos3–/– and wild-type C57BL/6J mice. Correlation analysis was performed by linear regression, followed by one-way ANOVA. Statistical significance was defined as P values less than .05. Values are reported as mean ± SE.
Results
Plasma tHcy, methionine, and folate
Plasma levels of tHcy were influenced significantly by both diet (P < .001) and Cbs genotype (P < .001), as shown in Figure 1A. Cbs+/+ mice had tHcy levels of 3.6 ± 0.2 μM when they were fed the control diet and 14.4 ± 1.1 μM when they were fed the HM/LF diet (P = .02). Cbs+/– mice had tHcy levels of 6.8 ± 0.5 and 43.3 ± 7.5 μM, respectively, when fed the control and HM/LF diets (P < .001). Plasma levels of methionine were influenced significantly by diet (P = .004). An effect of Cbs genotype on plasma methionine was observed only in mice fed HM/LF diet (P < .05; Figure 1B). Both Cbs+/+ and Cbs+/– mice fed the HM/LF diet had significantly lower levels of plasma folate than mice of the same genotypes fed the control diet (P < .001; Figure 1C).
Carotid artery thrombosis
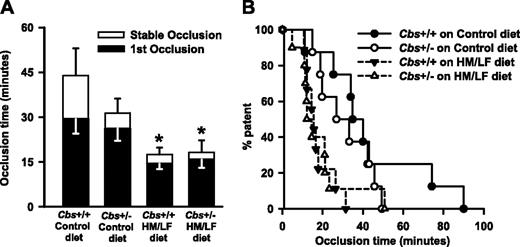
Experimental thrombosis of the carotid artery was induced by photochemical injury in mice that were anesthetized and ventilated mechanically to prevent respiratory acidosis.16 The mean time to stable occlusion did not differ significantly between Cbs+/+ and Cbs+/– mice fed the control diet (44.0 ± 9.1 versus 31.4 ± 4.7 minutes; P = .12) but was shortened by approximately 60% in both Cbs+/+ and Cbs+/– mice fed the HM/LF diet (17.5 ± 2.3 and 18.2 ± 4.0 minutes, respectively; P < .001; Figure 2A). The mean time to first occlusion also was significantly shorter in Cbs+/+ and Cbs+/– mice fed the HM/LF diet compared with Cbs+/+ mice fed the control diet (P < .001; Figure 2A). In both Cbs+/+ and Cbs+/– mice fed the HM/LF diet, 50% of animals exhibited stable occlusion of the carotid artery within 12 to 15 minutes of photochemical injury, compared with 35 minutes in Cbs+/+ mice fed the control diet and 27 minutes in Cbs+/– mice fed the control diet (Figure 2B).
Carotid artery thrombosis in Cbs+/+ and Cbs+/– mice. Carotid artery thrombosis following photochemical injury in Cbs+/+ and Cbs+/– mice fed either control or HM/LF diet (n = 8-10 in each group). (A) Time to first occlusion (▪) or stable occlusion (□). Values are mean ± SE. *P < .05 compared to mice of same genotype fed the control diet. (B) Percent of mice with a patent carotid artery as a function of time after administration of rose bengal.
Carotid artery thrombosis in Cbs+/+ and Cbs+/– mice. Carotid artery thrombosis following photochemical injury in Cbs+/+ and Cbs+/– mice fed either control or HM/LF diet (n = 8-10 in each group). (A) Time to first occlusion (▪) or stable occlusion (□). Values are mean ± SE. *P < .05 compared to mice of same genotype fed the control diet. (B) Percent of mice with a patent carotid artery as a function of time after administration of rose bengal.
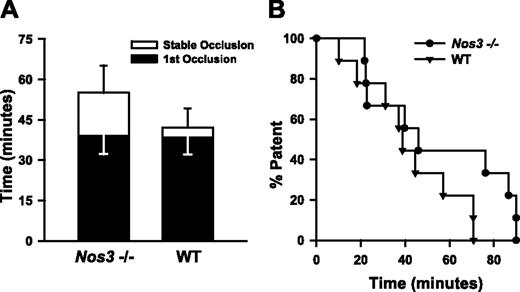
Because hyperhomocysteinemic mice have impaired NO-dependent endothelial function,14,23,26 we sought to determine whether loss of endothelium-derived NO can cause accelerated thrombosis. To examine this question, we measured susceptibility to carotid artery thrombosis in Nos3–/– mice. No significant differences in the mean times to first or stable occlusion were observed between Nos3–/– and wild-type C57BL/6 mice (Figure 3A), and the time at which 50% of animals exhibited stable occlusion was similar in Nos3–/– and wild-type mice (43 versus 40 minutes, P > .05). These findings suggest that lack of NO derived from endothelial NOS is unlikely be a sufficient mechanism to explain the accelerated thrombosis observed in Cbs+/+ or Cbs+/– mice with hyperhomocysteinemia.
Vascular ROS and NADPH oxidase
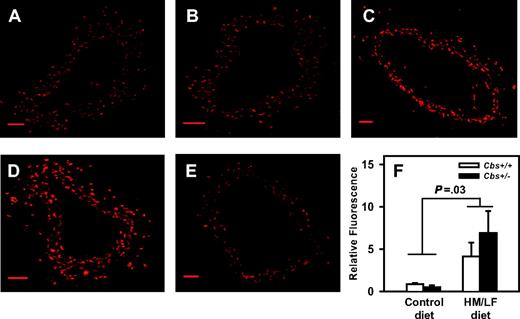
Production of ROS in the carotid artery was measured using the oxidative fluorescent dye, DHE. As observed previously,14 DHE fluorescence was detected at similar levels in sections of carotid artery from Cbs+/+ and Cbs+/– mice fed the control diet (Figure 4A-B). When Cbs+/+ or Cbs+/– mice were fed the HM/LF diet, however, a significant increase in DHE fluorescence was observed (P = .03 versus mice fed the control diet; Figure 4C-D,F). Preincubation of the carotid artery sections with PEG-SOD dramatically diminished DHE fluorescence in mice fed the HM/LF diet (Figure 4E), which suggests that a major part of the increased DHE fluorescence observed in these mice was due to superoxide.
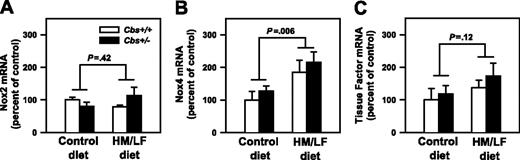
Previous studies have suggested that elevated production of vascular ROS in hyperhomocysteinemic animals can be attenuated by inhibitors of NADPH oxidases.14,27 To determine if hyperhomocysteinemia in mice alters the vascular expression of the catalytic subunits of NADPH oxidases, we measured mRNA levels of Nox1, Nox2, and Nox4 in the aorta. Levels of Nox1 mRNA were below the limit of detectability by real-time PCR in all groups of mice (data not shown). Nox2 mRNA was expressed at similar levels in Cbs+/+ and Cbs+/– mice fed either the control or HM/LF diets (Figure 5A). Nox4 mRNA was expressed at higher levels in Cbs+/+ and Cbs+/– mice that were fed the HM/LF diet than in those fed the control diet (P = .006; Figure 5B). By linear regression analysis, the levels of Nox4 mRNA in aorta correlated directly with plasma tHcy concentration (R = 0.40, P = .04).
Carotid artery thrombosis in Nos3–/– mice. Carotid artery thrombosis following photochemical injury in Nos3–/– mice (n = 9) compared with wild-type C57BL6/J mice (n = 10). All mice were fed the control diet. (A) Time to first occlusion (▪) or stable occlusion (□). Values are mean ± SE. (B) Percent of mice with a patent carotid artery as a function of time after administration of rose bengal.
Carotid artery thrombosis in Nos3–/– mice. Carotid artery thrombosis following photochemical injury in Nos3–/– mice (n = 9) compared with wild-type C57BL6/J mice (n = 10). All mice were fed the control diet. (A) Time to first occlusion (▪) or stable occlusion (□). Values are mean ± SE. (B) Percent of mice with a patent carotid artery as a function of time after administration of rose bengal.
Expression of tissue factor
Because homocysteine-induced oxidative stress has been proposed to up-regulate the expression of tissue factor,28,29 real-time PCR was performed to quantitatively measure levels of tissue factor mRNA in aorta. There was a nonsignificant trend toward increased tissue factor mRNA in the aorta of mice fed the HM/LF diet (P = .12; Figure 5C).
Activation of platelets
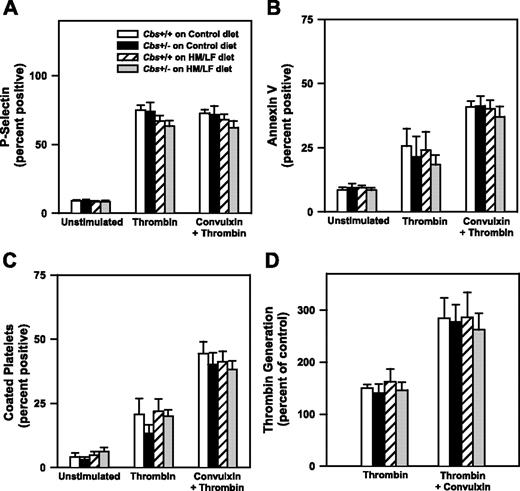
To further explore the potential mechanisms of accelerated thrombosis in hyperhomocysteinemic mice, we examined effects of hyperhomocysteinemia on platelet activation and the generation of anticoagulant protein C. To determine if platelets from hyperhomocysteinemic mice have altered platelet activation responses, platelets isolated from Cbs+/+ or Cbs+/– mice fed either the control or HM/LF diets were activated with thrombin in the absence or presence of the collagen receptor agonist, convulxin. The extent of platelet activation was assessed by flow cytometric measurement of surface P-selectin, annexin V binding, and formation of coated platelets, a highly activated subpopulation of platelets that retain high levels of surface fibrinogen.21 We also measured the procoagulant activity of activated platelets in a thrombin generation assay. As shown in Figure 6, dual stimulation with thrombin and convulxin produced greater levels of annexin V binding, coated platelets, and platelet procoagulant activity than stimulation with thrombin alone, but no significant effects of Cbs genotype or diet were observed for any of the platelet activation responses.
Representative laser-scanning confocal micrographs of carotid arteries stained with DHE. Sections of carotid artery were imaged with a laser scanning confocal microscope using a 10×/0.3 NA objective lens (BioRad, Hemel Hampstead, United Kingdom); images were acquired with BioRad Laser Sharp 2000 software. (A) Cbs+/+ mice fed the control diet. (B) Cbs+/– mice fed the control diet. (C) Cbs+/+ mice fed the HM/LF diet. (D) Cbs+/– mice fed the HM/LF diet. (E) Cbs+/– mice fed the HM/LF diet; sections preincubated with PEG-SOD. Scale bar represents 50 μM. (F) Relative DHE fluorescence (n = 6 in each group). Values are mean ± SEM.
Representative laser-scanning confocal micrographs of carotid arteries stained with DHE. Sections of carotid artery were imaged with a laser scanning confocal microscope using a 10×/0.3 NA objective lens (BioRad, Hemel Hampstead, United Kingdom); images were acquired with BioRad Laser Sharp 2000 software. (A) Cbs+/+ mice fed the control diet. (B) Cbs+/– mice fed the control diet. (C) Cbs+/+ mice fed the HM/LF diet. (D) Cbs+/– mice fed the HM/LF diet. (E) Cbs+/– mice fed the HM/LF diet; sections preincubated with PEG-SOD. Scale bar represents 50 μM. (F) Relative DHE fluorescence (n = 6 in each group). Values are mean ± SEM.
Activation of protein C
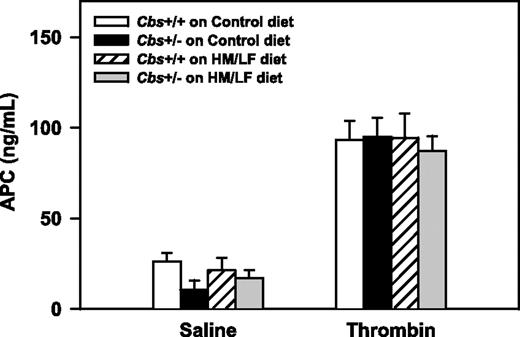
To determine if systemic activation of the anticoagulant, protein C, is altered in hyperhomocysteinemic mice in vivo, circulating levels of APC were measured after intravenous injection of either saline or thrombin. There were no significant effects of Cbs genotype or diet on basal (saline) or thrombin-stimulated levels of plasma APC (Figure 7). This finding suggests that hyperhomocysteinemia does not have a major effect on the activation of endogenous protein C in the pulmonary microcirculation, which is the primary site of protein C activation after intravenous infusion of thrombin.30
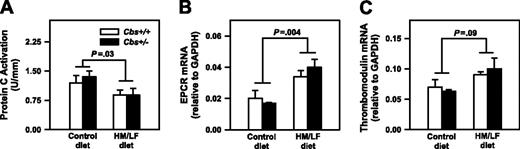
To determine if hyperhomocysteinemia influences protein C activation in large arteries, the activation of exogenous protein C by thrombin was measured using rings of proximal aorta. Activation of protein C in aorta was 30% lower in Cbs+/+ and Cbs+/– mice fed the HM/LF diet compared with the control diet (P = .03; Figure 8A). Because diminished protein C activation has been demonstrated to accelerate carotid artery thrombosis in other murine models,31 these findings suggest that local impairment of protein C activation in large arteries could contribute to the prothrombotic phenotype of hyperhomocysteinemic mice. To determine if hyperhomocysteinemia alters the expression of the endothelial cofactors for activation of protein C (thrombomodulin and EPCR),32 we also measured thrombomodulin and EPCR mRNA in the aorta by real-time PCR. Levels of EPCR mRNA were significantly higher in Cbs+/+ and Cbs+/– mice fed the HM/LF diet compared with the control diet (P = .004; Figure 8B), and a similar trend was observed for thrombomodulin mRNA (P = .09; Figure 8C). These findings suggest that mechanisms other than altered gene expression of thrombomodulin or EPCR, such as oxidative inactivation of thrombomodulin protein,33 may contribute to the decrease in protein C activation in hyperhomocysteinemic mice.
Levels of mRNA for Nox2, Nox4, and tissue factor. mRNA levels for (A) Nox2, (B) Nox4, and (C) tissue factor in the aorta, measured by real-time PCR (n = 6-7 in each group). The values were normalized to GAPDH mRNA using the comparative threshold cycle (ΔΔCT) method25 and are expressed as percent of the control value seen in Cbs+/+ mice fed the control diet. Values are mean ± SEM.
Levels of mRNA for Nox2, Nox4, and tissue factor. mRNA levels for (A) Nox2, (B) Nox4, and (C) tissue factor in the aorta, measured by real-time PCR (n = 6-7 in each group). The values were normalized to GAPDH mRNA using the comparative threshold cycle (ΔΔCT) method25 and are expressed as percent of the control value seen in Cbs+/+ mice fed the control diet. Values are mean ± SEM.
Discussion
The major findings of this study are: (1) mice with hyperhomocysteinemia exhibited enhanced susceptibility to carotid artery thrombosis; (2) acceleration of carotid artery thrombosis did not occur in mice deficient in endothelial NO synthase; and (3) accelerated thrombosis in hyperhomocysteinemic mice was associated with increased vascular oxidative stress and decreased activation of protein C in the aorta but normal platelet reactivity. Together, these findings suggest that hyperhomocysteinemia increases susceptibility to arterial thrombosis through a mechanism that is not mediated by loss of endothelium-derived NO or abnormal platelet activation but may involve oxidative stress and local impairment of the protein C anticoagulant pathway.
To induce thrombosis of the carotid artery, we chose to use a photochemical method of arterial injury that has been used previously to characterize susceptibility to thrombosis in several murine models.34 All mice were ventilated mechanically to avoid the hemodynamic consequences of altered cerebral blood flow that occur when anesthetized mice are not adequately ventilated.16 Accelerated carotid artery thrombosis was observed in both Cbs+/+ and Cbs+/– mice that were fed a diet rich in methionine and low in folate. As expected,23,35 the degree of hyperhomocysteinemia produced by the HM/LF diet was greater in Cbs+/– mice than in Cbs+/+ mice. The levels of plasma tHcy in mice with accelerated thrombosis (14.4 ± 1.1 and 43.3 ± 7.5 μM in Cbs+/+ and Cbs+/– mice, respectively) were within the range of plasma tHcy values seen in humans with moderate hyperhomocysteinemia and cardiovascular disease.8 The observation that the shortening of thrombotic occlusion times was nearly identical in Cbs+/+ and Cbs+/– mice fed the HM/LF diet suggests a threshold prothrombotic effect of elevated homocysteine. We have observed a similar threshold effect in previous studies of endothelial vasomotor dysfunction in hyperhomocysteinemic mice.14,15 It also is possible that the rose bengal photochemical thrombosis method may lack sensitivity to detect a further acceleration of thrombosis in mice with the highest levels of plasma tHcy. Alternatively, we cannot exclude the possibility that the prothrombotic effect of the HM/LF diet is mediated by a homocysteine-related metabolite such as methionine36 or folate.37,38
Platelet activation responses. Washed platelets from Cbs+/+ or Cbs+/– mice fed either the control or HM/LF diets were either left unstimulated or activated by thrombin (0.5 U/mL) or thrombin plus convulxin (250 ng/mL; n = 4-6 in each group). (A) Surface expression of P-selectin. (B) Annexin V binding. (C) Coated platelets, defined as the population of activated platelets with high surface levels of fibrinogen and low PE-JON/A binding.21 (D) Thrombin generation in a platelet procoagulant activity assay. Values are mean ± SEM.
Platelet activation responses. Washed platelets from Cbs+/+ or Cbs+/– mice fed either the control or HM/LF diets were either left unstimulated or activated by thrombin (0.5 U/mL) or thrombin plus convulxin (250 ng/mL; n = 4-6 in each group). (A) Surface expression of P-selectin. (B) Annexin V binding. (C) Coated platelets, defined as the population of activated platelets with high surface levels of fibrinogen and low PE-JON/A binding.21 (D) Thrombin generation in a platelet procoagulant activity assay. Values are mean ± SEM.
We considered several possible mechanisms of accelerated thrombosis in hyperhomocysteinemic mice, including effects on endothelium-derived NO, oxidative stress, platelet activation, and activation of protein C. Many previous studies have demonstrated that hyperhomocysteinemia causes endothelial dysfunction, with impaired bioavailability of endothelium-derived NO, in both humans39 and mice.10 We hypothesized, therefore, that loss of bioactive NO may promote thrombosis in mice by augmenting vasoconstriction, platelet activation, and oxidative stress. To test this hypothesis, we measured susceptibility to experimental thrombosis in Nos3–/– mice. Surprisingly, the time to occlusion of the carotid artery following photochemical injury was not shortened in Nos3–/– mice compared with age-matched wild-type control mice (Figure 3). These results demonstrate that a complete lack of Nos3 activity is not sufficient to accelerate carotid artery thrombosis in mice and further suggest that loss of endothelium-derived NO is unlikely to account for the accelerated thrombosis observed in Cbs+/+ or Cbs+/– mice fed the HM/LF diet. During the course of our studies, 2 other groups reported similar findings in Nos3–/– mice using a chemical method (ferric chloride) of arterial injury.40,41 The study by Iafrati et al further suggested that Nos3–/– mice have increased fibrinolytic activity that may compensate for a lack of vascular NO.40 Interestingly, homocysteine has been proposed to disrupt fibrinolytic pathways by inactivating annexin II42 and altering the structure of fibin clots,43 which raises the possibility that hyperhomocysteinemic mice may be unable to generate a compensatory increase in fibrinolysis in the setting of decreased endothelial NO.
Plasma levels of APC. Plasma levels of APC after intravenous injection of either saline or human thrombin (80 U/kg; n = 4-7 in each group). Values are mean ± SEM.
Plasma levels of APC. Plasma levels of APC after intravenous injection of either saline or human thrombin (80 U/kg; n = 4-7 in each group). Values are mean ± SEM.
In agreement with previous findings,37 we detected increased generation of ROS by DHE staining in the carotid arteries of Cbs+/+ and Cbs+/– mice fed HM/LF diet (Figure 4). Because NADPH oxidases have been suggested to be important sources of vascular ROS in hyperhomocysteinemia,27,37 we sought to determine if the vascular expression of any of the major catalytic subunits of NADPH oxidases (Nox1, Nox2 [also known as GP91phox], and Nox4)44 is altered in hyperhomocysteinemic mice. We found that Nox2 mRNA was expressed at similar levels in the aorta of mice with or without hyperhomocysteinemia but that Nox4 mRNA was expressed at significantly higher levels in hyperhomocysteinemic mice (Figure 5). These findings suggest that a Nox4-containing NADPH oxidase may contribute to increased vascular oxidative stress in hyperhomocysteinemic mice. As expected,44 levels of Nox1 mRNA were undetectable in the aorta of control mice. In contrast to previous findings in rat coronary arteries27 and cultured human microvascular endothelial cells,45 we did not detect an increase in Nox1 expression in the aorta of hyperhomocysteinemic mice. It is possible that there are species-specific differences in the effects of homocysteine on NADPH oxidase subunit expression in vascular cells, but we consider Nox4 to be an attractive target for future studies of homocysteine-induced oxidative stress in mice.
Protein C activation and EPCR and thrombomodulin mRNA levels. Activation of protein C (A) and levels of EPCR mRNA (B) and thrombomodulin mRNA (C) in the aorta of Cbs+/+ or Cbs+/– mice fed either the control or HM/LF diets (n = 6-7 in each group). Levels of EPCR mRNA and thrombomodulin mRNA were normalized to levels of GAPDH mRNA. Values are mean ± SEM.
Protein C activation and EPCR and thrombomodulin mRNA levels. Activation of protein C (A) and levels of EPCR mRNA (B) and thrombomodulin mRNA (C) in the aorta of Cbs+/+ or Cbs+/– mice fed either the control or HM/LF diets (n = 6-7 in each group). Levels of EPCR mRNA and thrombomodulin mRNA were normalized to levels of GAPDH mRNA. Values are mean ± SEM.
We considered the possibility that hyperhomocysteinemia-induced ROS may lead to the up-regulation of the procoagulant tissue factor in vascular tissue. Tissue factor expression is redox-sensitive in many cell types, including endothelial and smooth muscle cells,46 and some studies have reported up-regulation of tissue factor by homocysteine.10 We found only nonsignificant increases in tissue factor mRNA in the aorta of Cbs+/+ and Cbs+/– mice fed the HM/LF diet (Figure 5C), which suggests that oxidative up-regulation of tissue factor expression in the vascular wall is unlikely to be a major contributor to accelerated thrombosis in hyperhomocysteinemic mice.
We also considered the possibility that hyperhomocysteinemia may increase platelet activation, perhaps through an oxidative mechanism.47 We did not, however, observe any effects of hyperhomocysteinemia on platelet activation responses (surface P-selectin, annexin V binding, coated platelet formation, or platelet procoagulant activity) after activation with thrombin or thrombin plus convulxin (Figure 6). A limitation of these results is that platelet activation was examined in vitro using washed platelets, so the possibility that hyperhomocysteinemic mice may have enhanced platelet activation in vivo cannot be excluded.
To further explore the mechanisms of accelerated thrombosis in hyperhomocysteinemic mice, we measured the activation of anticoagulant protein C. In the presence of thrombin and 2 endothelial cofactors, thrombomodulin and EPCR, protein C is converted to its activated form (APC).32 APC exerts its anticoagulant activity by cleaving activated coagulation factors Va and VIIIa. In previous studies, we found that activation of protein C was decreased in the aorta of hyperhomocysteinemic monkeys48 and mice,23 but systemic activation of protein C in response to infusion of thrombin was preserved in monkeys with mild hyperhomocysteinemia.30 In agreement with these previous findings, we did not detect a significant effect of hyperhomocysteinemia on systemic activation of protein C, but we did find a 30% decrease in protein C activation in the aorta of Cbs+/+ and Cbs+/– mice fed the HM/LF diet compared with mice fed the control diet (Figure 8).
We next tested the hypothesis that hyperhomocysteinemia causes down-regulation of thrombomodulin or EPCR mRNA. In the aorta, decreased protein C activation was accompanied by increased mRNA levels for both EPCR and thrombomodulin in mice fed HM/LF diet (Figure 8B-C). These findings suggest that decreased protein C activation in the aorta of hyperhomocysteinemic mice is not caused by decreased gene expression of EPCR or thrombomodulin. Instead, the results suggest that hyperhomocysteinemia may be associated with compensatory up-regulation of thrombomodulin and EPCR expression in the aorta. We conclude that mechanisms other than altered thrombomodulin and EPCR gene expression, such as oxidative inactivation of thrombomodulin protein,33 likely contribute to decreased protein C activation in hyperhomocysteinemic mice.
In summary, the results reported here demonstrate for the first time that hyperhomocysteinemia accelerates carotid artery thrombosis in mice. The degree of elevation of plasma tHcy associated with enhanced susceptibility to thrombosis in mice is quite similar to that seen in humans with mild or moderate hyperhomocysteinemia. The mechanism of accelerated thrombosis in hyperhomocysteinemic mice appears to be unrelated to loss of endothelium-derived NO, and we did not observe any significant effects of hyperhomocysteinemia on platelet activation. Instead, our data suggest that the prothrombotic effects of hyperhomocysteinemia may be related to increased vascular oxidative stress or decreased protein C activation in large arteries. Future studies are needed to more clearly define the mechanistic role of the NADPH oxidase catalytic subunit Nox4 and the relationship between vascular ROSs, the activity and expression of thrombomodulin, EPCR, and tissue factor, and other procoagulant mechanisms.
Prepublished online as Blood First Edition Paper, June 26, 2006; DOI 10.1182/blood-2006-02-005991.
Supported by the Office of Research and Development, Department of Veterans Affairs; National Institutes of Health grants HL63943, NS24621, and HL62984; and an American Heart Association Postdoctoral Fellowship Award (S.D.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Sara Rozen and Kevin L. Knudson for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal