Abstract
Autoimmune lymphoproliferative syndrome (ALPS) is a disorder of abnormal lymphocyte survival caused by defective Fas-mediated apoptosis, leading to lymphadenopathy, hepatosplenomegaly, and an increased number of double-negative T cells (DNTs). Treatment options for patients with ALPS are limited. Rapamycin has been shown to induce apoptosis in normal and malignant lymphocytes. Since ALPS is caused by defective lymphocyte apoptosis, we hypothesized that rapamycin would be effective in treating ALPS. We tested this hypothesis using rapamycin in murine models of ALPS. We followed treatment response with serial assessment of DNTs by flow cytometry in blood and lymphoid tissue, by serial monitoring of lymph node and spleen size with ultrasonography, and by enzyme-linked immunosorbent assay (ELISA) for anti–double-stranded DNA (dsDNA) antibodies. Three-dimensional ultrasound measurements in the mice correlated to actual tissue measurements at death (r = .9648). We found a dramatic and statistically significant decrease in DNTs, lymphadenopathy, splenomegaly, and autoantibodies after only 4 weeks when comparing rapamycin-treated mice with controls. Rapamycin induced apoptosis through the intrinsic mitochondrial pathway. We compared rapamycin to mycophenolate mofetil, a second-line agent used to treat ALPS, and found rapamycin's control of lymphoproliferation was superior. We conclude that rapamycin is an effective treatment for murine ALPS and should be explored as treatment for affected humans.
Introduction
Patients with autoimmune lymphoproliferative syndrome (ALPS) typically present in early childhood with lymphadenopathy and organomegaly, and many patients develop autoimmune cytopenias and secondary neoplasms. ALPS is thought to be a rare condition1 ; however, we have demonstrated in a single institution study that ALPS is likely more common than previously thought.2 Treatments for patients with ALPS are limited to nonspecific immune modulators with extensive side-effect profiles.3 The pathophysiology of ALPS has been partially characterized allowing for the rational evaluation of potential treatments.
ALPS is caused by defects in the Fas apoptotic pathway, suggesting agents that can induce lymphocyte apoptosis may be useful treatments. Rapamycin is an immunosuppressive and antineoplastic agent with a tolerable side-effect profile, belonging to a class of drugs that inhibit the mammalian target of rapamycin (mTOR).4 These mTOR inhibitors may induce apoptosis in T and B lymphocytes.5,6 We hypothesized that rapamycin would be effective in reducing symptoms and treating the disease in patients with ALPS by inducing apoptosis in abnormal B and T lymphocytes. Rapamycin has been studied in other illnesses and in pediatric patients4 ; however, prior to testing any novel therapy in ALPS patients, it is important to establish efficacy in animal models.
Most patients with ALPS have mutations in the FAS gene.7 Multiple murine models with different backgrounds exist with FAS mutations; however, CBA-lprcg has been shown to have a phenotype and genotype most similar to most patients with ALPS.1 We studied the efficacy of rapamycin in CBA-lprcg mice and established the activity of this agent against ALPS. We also studied the efficacy of rapamycin against autoimmunity using a similar mouse model, MRL-lpr. We used high-frequency small-animal ultrasonography to follow response to the drug and this is the first report we are aware of to use ultrasound to follow a nonmalignant murine disease.
Materials and methods
Animals and rapamycin treatment schedule
CBA-lprcg mice aged 5 to 6 months (Jackson Laboratories, Bar Harbor, ME) were randomized (1:1) to treatment with rapamycin (Wyeth Ayerst Laboratories, Marietta, PA) or vehicle after establishment of clinically identifiable lymphadenopathy, defined as having at least 1 palpable lymph node greater than 100 mm3 in size as assessed by high-frequency small-animal ultrasonography (see “Assessment of response to rapamycin treatment: ultrasound measurement of lymph node and spleen size”). Rapamycin was given at a dose of 5 mg/kg/day, 5 days a week.6 Mice were treated in 2 cohorts. The first cohort of 4 mice (2 treatment, 2 control) was treated for 6 weeks and killed for tissue analysis. In the second cohort, 11 mice (6 treatment, 5 control) were treated for 3 months and then killed.
Assessment of response to rapamycin treatment: ultrasound measurement of lymph node and spleen size
High-frequency small-animal ultrasonography is used as a novel tool for measuring changes in organ or tumor size with disease progression or in response to drug treatment.8 Ultrasound imaging was performed using a Vevo 660 small-animal ultrasound (VisualSonics, Toronto, ON, Canada). This model ultrasound was recently demonstrated to be reproducibly accurate in 3-D assessment of intra-abdominal tumor size in a murine pancreatic cancer model.8 We used the Vevo 660 to follow lymph node volume and splenic area. Prior to using the ultrasound to follow disease burden in these experiments, measurements from our machine were validated by comparing measured organ sizes to actual sizes by serial killing of multiple strains of mice and evaluation of multiple tissues (N.R. and J.M.M., unpublished data, 2004-2005).
Prior to the day of ultrasound, abdominal hair was removed from the mice using a depilatory cream. Mice were anesthetized using isofluorane. We used a 40-MHz center frequency probe for our measurements that has a fixed focal depth of 6 mm and resolution to 30 μm. B-mode imaging was used to obtain 2-D dynamic images of mouse lymph nodes and spleens. Three-dimensional images were collected of lymph nodes by a motorized drive that collects regular spatial images through a tissue at 30-μm slices. Data were stored and analyzed using Visualsonics software, version 1.3.8.
Mice were randomized to rapamycin or vehicle after they developed at least 1 palpable lymph node. Ultrasound assessment of lymph node size was performed the day mice were enrolled onto study and started drug treatment. Only mice with at least 1 lymph node of 100 mm3 in volume as assessed by ultrasound were enrolled, and we followed the largest lymph node at initiation of treatment in each mouse. Upon study entry and every 2 weeks until death, 1 lymph node was serially measured for volume by 3-D imaging, and splenic length and height were measured by B-mode imaging. Comparisons were made between treated and untreated animals by 2-sided t test. We compared actual size of spleen and lymph nodes by caliper measurements to ultrasound measurements at death to validate measurements. All data analysis and ultrasound measurements were blinded as to treatment arm.
Assessment of response to rapamycin treatment: peripheral blood for DNTs
Patients and mice with ALPS have an increased number of an unusual T-cell population, double-negative T cells (DNTs; T-cell phenotype CD3+, CD4–, CD8–, T-cell receptor [TCR]αβ+)9 in both peripheral blood and lymphoid tissues. We followed blood counts and DNTs in the mice by retro-orbital bleed at initiation of treatment and serially every other week. Comparisons were made between treated and untreated by 2-sided t test. Complete blood count (CBC) analysis was performed on a HemeVet 850FS hematology analyzer (CDC Technologies, Oxford, CT), and flow cytometric analysis for DNTs was performed using anti–TCRβ chain–FITC, anti–CD4-PE, anti-CD8a (Ly-2)–PerCP-CY5.5, and anti–CD3ϵ-APC-Cy7. All antibodies were purchased from BD Pharmigen (Franklin Lakes, NJ). We monitored absolute DNT count, defined as white blood cells (WBCs; measured by HemeVet hematology analyzer) × % lymphocytes (calculated by HemeVet hematology analyzer and confirmed by forward scatter/side scatter [FSC/SSC] on flow cytometry and manual differential on peripheral smear) × % DNTs (calculated by flow cytometry).
Comparison of rapamycin to mycophenolate mofetil
Mycophenolate mofetil (MMF; F. Hoffmann-La Roche, Basel, Switzerland) is an immunosuppressant that has been used to treat patients with ALPS.10 We compared the activity of rapamycin to MMF in our murine ALPS model. We randomized mice to 2:2:1 with rapamycin, MMF, or vehicle control after development of identifiable lymphadenopathy as described in “Animals and rapamycin treatment schedule.” Five mice were treated with rapamycin at 5 mg/kg/day and 5 mice were treated with MMF at 100 mg/kg/day intraperitoneally.11,12 Nine mice received no treatment, representing a cohort from this randomization plus historical controls from the earlier experiments described in this work. Mice were treated for 3 months and lymphadenopathy, splenomegaly, and peripheral blood DNTs were followed serially as described in “Assessment of response to rapamycin treatment: peripheral blood for DNTs.”.
Assessment of autoantibodies
MRL-lpr mice (Jackson Laboratories) were randomized (1:1:1) to treatment with rapamycin, MMF, or vehicle control beginning at 3 months of age. Four mice were treated with rapamycin at a dose of 5 mg/kg/day by gavage, 4 mice were treated with MMF at a dose of 100 mg/kg/day intraperitoneally, and 4 mice received no treatment. Mice were treated for 4 weeks, and serum was obtained from mice by retro-orbital bleed at initiation of treatment and serially every other week. Serum samples were frozen at collection and all samples were analyzed together for mouse anti–double-stranded DNA (dsDNA) immunoglobulin G (IgG)–specific antibodies using a 96-well quantitative enzyme-linked immunosorbent assay (ELISA) kit from Alpha Diagnostic International (San Antonio, TX). All samples were run in duplicate as recommended in the manufacturer's instructions, and 1 μL sera was used for each well.
Immunoblotting
Immunoblots were performed to detect the mTOR pathway target S6 in order to assess inhibition of this pathway, and to detect the phosphorylation of the proapoptotic molecule Bad. For these experiments, we used our published methods13 based on a modification of the protocol of Huang et al.14 In brief, mouse lymph nodes were harvested and dissociated into a single cell suspension. Cells (10-20 × 106) were lysed in ice-cold lysis buffer, and nuclei were sedimented from the lysate. Equal quantities of protein (20-40 μg) from each lysate were resolved using NuPAGE Tris-bis SDS gel electrophoresis (10% SDS for S6 and 12% SDS for Bad; Cell Signaling Technologies, Beverly, MA), transferred to PVDF membranes (PerkinElmer Life Sciences, Boston, MA), and labeled with antibodies to S6, phospho-S6 (ser235/ser236), Bad, and phospho-Bad (Ser 112 and Ser 136) (Cell Signaling Technologies) for 18 hours at 4°C and detected using a horseradish peroxidase (HRP)–conjugated goat antirabbit secondary antibody (Jackson ImmunoResearch laboratories, West Grove, PA) and the Lumiglo chemiluminescence system (Cell Signaling Technologies). Bands were outlined and quantitated densitometrically using Quantity-One software (Bio-Rad, Hercules, CA).
In vitro assessment of apoptosis
T lymphocytes from CBA-lprcg mice were sustained in vitro for apoptosis testing using published techniques.15 In brief, lymph nodes were harvested from 5- to 6-month-old mice. Cells were maintained at 37°C under 5% CO2 in RPMI 1640 media with 10% heat inactivated fetal calf serum, 10 mM HEPES (pH 7.5), 1 mM sodium pyruvate, 100 μM nonessential amino acids, 100 U/mL penicillin, 100 μg/mL streptomycin, 50 μM 2-mercaptoethanol (Fisher Scientific, Hampton, NH), and 10 U/mL murine IL-2 (Lenico, St Louis, MO). After 7 days in culture, viable cells were isolated by Ficoll-Isopaque and expanded. This methodology can successfully support and maintain CD3+CD4+ and CD3+CD8+ T lymphocytes from the mice. DNTs do not survive in culture.15 Seven days later, aliquots of 105 lymphocytes were exposed to 100 ng/mL rapamycin for 48 hours and were assessed for apoptosis by flow cytometric staining for annexin V–FITC (BD Pharmigen) and 7-aminoactinomycin-D (7-AAD; BD Pharmigen). Apoptotic cells are annexin V positive and 7-AAD negative. In order to determine if apoptosis was caspase dependent, prior to treating with rapamycin, aliquots of cells were pretreated for 2 hours with 50 μM pancaspase inhibitor (Z-VAD-FMK), 50 μM caspase-9 inhibitor (Z-LEHD-FMK), or 50 μM caspase-8 inhibitor (Z-IETD-FMK). Caspase inhibitors were all purchased from Biovision Research Products (Mountain View, CA). Cells were also treated with 5 nM etoposide (Sicor, Irvine, CA) as a positive control for apoptosis.
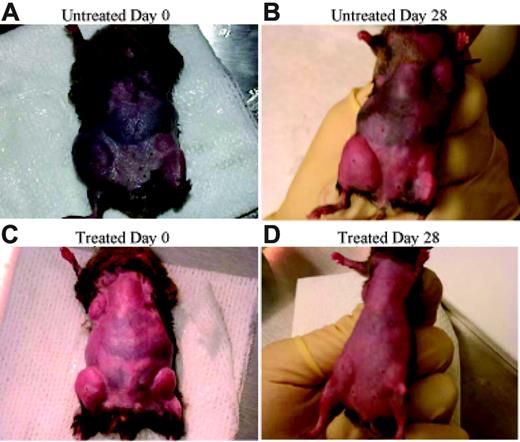
Rapamycin decreases lymphoproliferation. CBA-lprcg mice were randomized to treatment with rapamycin versus vehicle control. After 4 weeks of treatment a decrease in adenopathy is visually apparent.
Rapamycin decreases lymphoproliferation. CBA-lprcg mice were randomized to treatment with rapamycin versus vehicle control. After 4 weeks of treatment a decrease in adenopathy is visually apparent.
Results
Rapamycin decreases lymphoproliferation in murine ALPS
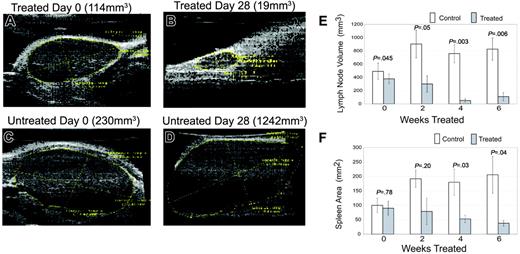
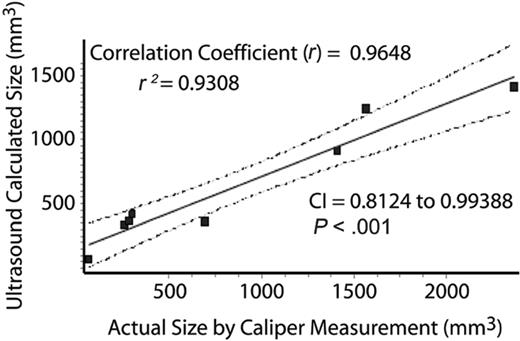
We found a striking decrease in lymphoproliferation in the mice after treatment with rapamycin (Figure 1). At initiation of treatment, average size of lymph nodes and spleen were not statistically different between treated and control group as assessed by ultrasound measurement (Figure 2). As early as 2 weeks after initiating treatment, rapamycin-treated mice had a statistically significant decrease in lymph node size (Figure 2), and by 4 weeks after initiating treatment, rapamycin-treated mice had a statistically significant decrease in spleen size (Figure 2). We compared actual size of lymph nodes (Figure 3; correlation coefficient r = .96, P < .001) and spleen (Figure S1 [available at the Blood website; see the Supplemental Figures link at the top of the online article]; correlation coefficient r = .94, P < .001) to ultrasound measurements obtained on day of death and found correlation between measurements. These results demonstrate that rapamycin decreases both lymphadenopathy and splenomegaly in murine ALPS, and that small-animal ultrasound provides an accurate tool to measure lymphoproliferation in mice.
Rapamycin decreases lymphoproliferation. (A-D) Serial ultrasounds were performed every 2 weeks to document lymph node volume in cubic millimeters and splenic area in square millimeters, comparing rapamycin-treated with untreated mice. Treated mice showed a statistically significant (P = .05) decrease in lymph node volume after 2 weeks of treatment when compared with control mice (E). Treated mice also showed a statistically significant (P = .03) decrease in splenic area by 4 weeks of treatment when compared with control mice (F). No statistical difference existed between groups at initiation of treatment. Bars represent mean lymph node volume or splenic areas from mice at each time point and error bars represent SEM. Average normal mouse spleen size in unaffected animals was 10 to 20 mm2. (A) Representative example of a lymph node prior to treatment with rapamycin. (B) The same node from panel A after 4 weeks of treatment. Initial volume of the node was 114 mm3 and the volume after treatment was 19 mm3, representing an 80% reduction in size. (C) In contrast to panels A-B, a lymph node of a mouse prior to treatment with vehicle. (D) The same node from panel C after 4 weeks. Initial volume of this node was 230 mm3 and the volume after exposure to vehicle was 1242 mm3, representing a 600% increase in size.
Rapamycin decreases lymphoproliferation. (A-D) Serial ultrasounds were performed every 2 weeks to document lymph node volume in cubic millimeters and splenic area in square millimeters, comparing rapamycin-treated with untreated mice. Treated mice showed a statistically significant (P = .05) decrease in lymph node volume after 2 weeks of treatment when compared with control mice (E). Treated mice also showed a statistically significant (P = .03) decrease in splenic area by 4 weeks of treatment when compared with control mice (F). No statistical difference existed between groups at initiation of treatment. Bars represent mean lymph node volume or splenic areas from mice at each time point and error bars represent SEM. Average normal mouse spleen size in unaffected animals was 10 to 20 mm2. (A) Representative example of a lymph node prior to treatment with rapamycin. (B) The same node from panel A after 4 weeks of treatment. Initial volume of the node was 114 mm3 and the volume after treatment was 19 mm3, representing an 80% reduction in size. (C) In contrast to panels A-B, a lymph node of a mouse prior to treatment with vehicle. (D) The same node from panel C after 4 weeks. Initial volume of this node was 230 mm3 and the volume after exposure to vehicle was 1242 mm3, representing a 600% increase in size.
Rapamycin decreases DNTs in murine ALPS
Patients with ALPS have an increased number of an atypical T-cell population, DNTs (T-cell phenotype CD3+, CD4–, CD8–, TCRαβ+),9 that represent less than 1% of the T-cell population in healthy individuals.16 DNTs are elevated in both peripheral blood and lymphoid tissues in patients with ALPS. These cells express both the TCR and CD3, yet lack expression of CD4 and CD8. These cells may represent CD8+ cytotoxic T lymphocytes that lose CD8 expression,16 or a subset of regulatory T (Treg) cells.17
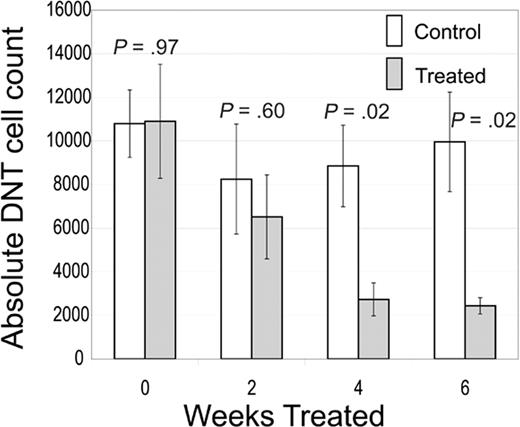
CBA-lprcg mice also develop elevated DNTs in blood and lymphoid tissue.18 We followed blood counts and DNTs in the mice by serial sampling of peripheral blood and found a statistically significant decrease in peripheral blood absolute DNTs by 4 weeks of treatment with rapamycin (Figure 4). We found the decrease in DNTs was durable even in a cohort treated for 3 months. This decrease in DNTs was also observed in lymphoid tissues, including spleens (average number in treated mice, 57 × 106 [range, 30 × 106–73 × 106] versus control, 158 × 106 [range, 48 × 106–345 × 106]; P = .04) and lymph nodes (average number in treated mice, 7 × 106 [range, 0-27 × 106] versus control, 142 × 106 [range, 67 × 106–316 × 106]; P = .009).
Ultrasound accurately estimates organ volume. In order to ensure that ultrasound measurements reflected actual measurements, lymph node volumes from CBA-lprcg mice at death (calculated as 4/3π × radius height × radius width × radius length of lymph node using caliper) were compared with volume calculated on Vevo 660 ultrasound. Linear regression analysis demonstrated a statistically significant correlation. Dotted lines indicate 95% confidence intervals.
Ultrasound accurately estimates organ volume. In order to ensure that ultrasound measurements reflected actual measurements, lymph node volumes from CBA-lprcg mice at death (calculated as 4/3π × radius height × radius width × radius length of lymph node using caliper) were compared with volume calculated on Vevo 660 ultrasound. Linear regression analysis demonstrated a statistically significant correlation. Dotted lines indicate 95% confidence intervals.
These results demonstrate that rapamycin decreases DNTs, a surrogate marker of disease in murine ALPS.
Toxicity profile of rapamycin in murine ALPS
No toxicities were noted in mice treated with rapamycin. No difference was noted in hemoglobin or platelet count between groups and no mice developed anemia or thrombocytopenia. No mouse developed any apparent infection or other clinical manifestation of immunosuppression during treatment with rapamycin. The percentage of CD3/4+ and CD3/8+ T cells increased in the lymph nodes of rapamycin-treated animals as the percentage of DNTs decreased. Because there was a significant reduction in size and cell number of lymph node and spleen in treated animals, absolute CD3/CD4 counts in lymph node and spleen were reduced in treated animals (lymph node, treated average 15 × 106 and untreated control average 41 × 106, P = .03; spleen, treated average 28 × 106 and untreated control average 48 × 106, P = .04). Absolute CD3/CD8 counts were also reduced; however, the reduction was not statistically significant.
Rapamycin superior to MMF in murine ALPS
MMF has been proposed as a treatment for patients with ALPS.10 We therefore compared treatment with rapamycin to MMF in these CBA-lprcg mice. We found rapamycin treatment resulted in superior responses against lymphoproliferation compared with MMF treatment. As we found in our earlier experiments, rapamycin-treated mice had a statistically significant decrease in lymphadenopathy, splenomegaly, and DNTs (data not shown). Mice treated with MMF had disease stabilization only for 4 to 6 weeks followed by progression (Figure 5 and Figure S2). Mice treated with MMF did have an initial trend toward reduction in DNTs (P = .07) when compared with the control at 6 weeks; however, mice then progressed (Figure S2). When comparing rapamycin with MMF we found a statistically significant reduction in lymphadenopathy (P = .04) and splenomegaly (P = .02) after 6 weeks in the rapamycin-treated mice (Figures 5, S2). We found rapamycin reduced DNTs to a greater degree than did MMF; however, the difference was not statistically significant (Figure S2). These data indicate that rapamycin was superior to MMF in this murine model of ALPS.
Rapamycin decreases DNTs. CBA-lprcg mice were randomized to treatment with rapamycin 5 mg/kg/day versus vehicle control. Retro-orbital bleeds were performed every 2 weeks to assess absolute DNTs/mm3. Treated mice showed a statistically significant decrease in absolute DNTs compared with control by 4 weeks of treatment. No statistical difference existed between groups at initiation of treatment. Bars represent mean absolute DNT count from mice at each time point; error bars represent SEM. Normal mouse absolute DNT count range, 30 to 150/mm3.9,18
Rapamycin decreases DNTs. CBA-lprcg mice were randomized to treatment with rapamycin 5 mg/kg/day versus vehicle control. Retro-orbital bleeds were performed every 2 weeks to assess absolute DNTs/mm3. Treated mice showed a statistically significant decrease in absolute DNTs compared with control by 4 weeks of treatment. No statistical difference existed between groups at initiation of treatment. Bars represent mean absolute DNT count from mice at each time point; error bars represent SEM. Normal mouse absolute DNT count range, 30 to 150/mm3.9,18
Assessment of autoantibodies
Although the CBA-lprcg mouse is felt to be the most similar to human ALPS, these mice develop only minimal autoantibodies.1 We used a different murine ALPS model, MRL-lpr, to assess the effect of rapamycin and MMF on murine autoantibodies. We measured mouse anti-dsDNA IgG-specific antibodies in the mice using a quantitative ELISA. We found rapamycin substantially reduced the level of autoantibodies in the mice after only 2 weeks of treatment (Figure 6). While there was no statistical difference between titer levels in the 3 groups at initiation of treatment, we found a statistically significant difference in average titer levels comparing rapamycin with MMF (0.3 μg/mL vs 5 μg/mL; P < .001) and rapamycin with control (0.3 μg/mL vs 8 μg/mL; P = .001) after 4 weeks of treatment. We found MMF did not decrease antibody production, but did stabilize levels in some of the animals, consistent with other studies that demonstrated MMF has little effect on autoantibodies in MRL-lpr mice.19
Evaluation of mTOR signaling pathway by immunoblot
We hypothesized that the clinical response to rapamycin would be associated with a biochemical response, indicating down-regulation of the mTOR signaling pathway. We have previously shown that activation of S6K1 (p70S6 kinase), an mTOR pathway intermediate, is down-regulated by rapamycin in murine B-cell lines.6 Similarly, other groups have assessed phosphorylation of S6, the target of S6K1, in normal cells as a marker of mTOR inhibition.20 When mTOR is active, S6K1 phosphorylates S6, leading to ribosomal protein synthesis. Inhibition of mTOR results in dephosphorylation of S6 and inhibits this process. We performed immunoblots on lymph node cells obtained from treated and control mice to assess phosphorylation of S6. In order to compare treated with untreated cells in the same mouse, we also removed a lymph node from a mouse prior to rapamycin treatment and removed a second lymph node from the same mouse after 3 days of treatment and assessed phosphorylation of S6. We were able to show down-regulation of the mTOR pathway as a consequence of rapamycin treatment (Figure 7). Immunoblot analysis of cell lysates from rapamycin-treated mice demonstrated a 40% to 62% decrease in the amount of phospho-S6 compared with cells from untreated mice. These results demonstrate that the clinical response we found in the mice is associated with a biochemical response in the mTOR signaling pathway.
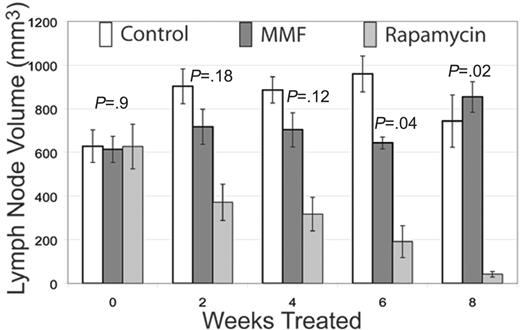
Rapamycin is superior to MMF. CBA-lprcg mice were randomizd to treatment with rapamycin, MMF, or control. Serial ultrasounds were performed every 2 weeks to document lymph node volume in cubic millimeters. Rapamycin-treated mice showed a statistically significant (P = .04) decrease in lymph node volume after 6 weeks of treatment when compared with MMF-treated mice. Bars represent mean lymph node volume from mice at each time point; error bars represent SEM. P values depict comparisons of rapamycin and MMF treatment by 2-tailed t test.
Rapamycin is superior to MMF. CBA-lprcg mice were randomizd to treatment with rapamycin, MMF, or control. Serial ultrasounds were performed every 2 weeks to document lymph node volume in cubic millimeters. Rapamycin-treated mice showed a statistically significant (P = .04) decrease in lymph node volume after 6 weeks of treatment when compared with MMF-treated mice. Bars represent mean lymph node volume from mice at each time point; error bars represent SEM. P values depict comparisons of rapamycin and MMF treatment by 2-tailed t test.
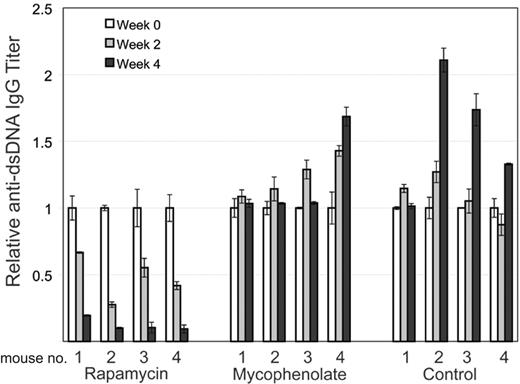
Rapamycin decreases autoantibody production. MRL-lpr mice were randomized to treatment with rapamycin, MMF, or control. Retro-orbital bleeds were obtained every 2 weeks to measure mouse anti-dsDNA IgG-specific antibodies in sera by quantitative ELISA. Mice treated with rapamycin had a statistically significant decrease in average titer levels compared with MMF (0.3 μg/mL vs 5 μg/mL; P < .001) and control (0.3 μg/mL vs 8 μg/mL; P = .001) after 4 weeks of treatment. Figure 6 depicts results of antibody titers for each mouse over time with 4 mice in each treatment group. Results are normalized to titer at initation of treatment for each mouse. Error bars represent SEM.
Rapamycin decreases autoantibody production. MRL-lpr mice were randomized to treatment with rapamycin, MMF, or control. Retro-orbital bleeds were obtained every 2 weeks to measure mouse anti-dsDNA IgG-specific antibodies in sera by quantitative ELISA. Mice treated with rapamycin had a statistically significant decrease in average titer levels compared with MMF (0.3 μg/mL vs 5 μg/mL; P < .001) and control (0.3 μg/mL vs 8 μg/mL; P = .001) after 4 weeks of treatment. Figure 6 depicts results of antibody titers for each mouse over time with 4 mice in each treatment group. Results are normalized to titer at initation of treatment for each mouse. Error bars represent SEM.
Assessment of mechanism of response to rapamycin
We hypothesized that rapamycin would be effective in ALPS by inducing apoptosis in abnormal T lymphocytes. We examined lymphocyte apoptosis in vivo by assessing phosphorylation status of the proapoptotic molecule Bad, and we examined lymphocyte apoptosis in vitro by flow cytometric analysis for annexin V of rapamycin-treated lymphocytes derived from CBA-lprcg mice. Further, we pretreated the lymphocytes with 3 different caspase inhibitors to determine if the apoptosis was caspase dependent and whether the apoptosis was intrinsic to the mitochondrial apoptotic pathway.
Bad is a Bcl2 family, proapoptotic molecule that is regulated by phosphorylation. When Bad is phosphorylated it is then sequestered by 14-3-3 proteins and rendered inactive, providing a survival signal to cells. In contrast, when Bad is dephosphorylated, it provides proapoptotic effects on cells through the intrinsic mitochondrial pathway.21 Prior work has shown that mTOR inhibitors, including rapamycin, lead to the desphosphorylation of Bad with subsequent apoptosis.22 We harvested lymph node cells from CBA-lprcg mice treated with rapamycin and from control mice to assess phosphorylation of Bad. We were able to show decreased phosphorylated Bad in rapamycin-treated mice (Figure 7), suggesting activation of the intrinsic mitochondrial pathway. Immunoblot analysis of cell lysates from rapamycin-treated mice demonstrated a more than 80% reduction in phospho-Bad compared with cells from treated mice.
We also treated cultured T lymphocytes obtained from harvested mouse lymph nodes with rapamycin. We found rapamycin induced apoptosis in the cultured T lymphocytes, and this apoptosis could be significantly but not completely blocked with the addition of a caspase-9 or pancaspase inhibitor, but could not be blocked with the addition of a caspase-8 inhibitor (Figure 7). These results suggest rapamycin works, at least in part, through a caspase-dependent apoptotic mechanism and primarily targets the intrinsic mitochondrial apoptotic pathway.
Discussion
Rapamycin, an mTOR inhibitor, is both an immunosuppressant and antineoplastic agent.6,23,24 Rapamycin is US Food and Drug Administration (FDA) approved as an immunosuppressive agent in solid organ transplantation.24,25 It has documented efficacy in autoimmune diseases, including rheumatoid arthritis.23 Rapamycin has been shown to induce apoptosis in B and T lymphocytes.5,6 mTOR inhibitors are active in models of Epstein-Barr virus (EBV) lymphoproliferative disease,26 and we have demonstrated that rapamycin and other drugs that target the mTOR pathway are effective against lymphoid malignancies.6,13 Since ALPS is caused by defective lymphocyte apoptosis, we hypothesized that rapamycin would be an effective agent in ALPS by inducing apoptosis in the defective lymphocytes. We demonstrated that rapamycin is very effective in reducing disease burden in murine ALPS, and we have evidence that suggests that the mechanism of that response was through caspase-dependent apoptotic induction through the intrinsic mitochondrial pathway.
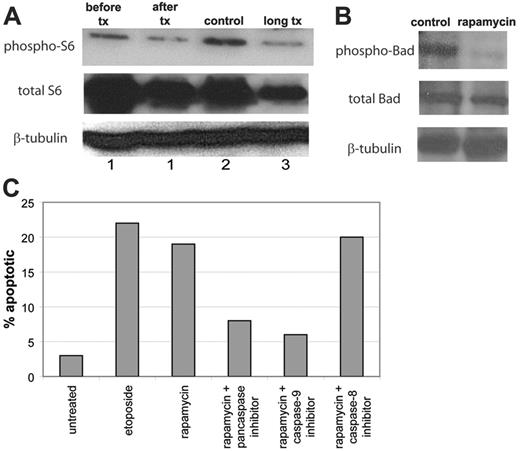
Mechanism of action of rapamycin in ALPS. (A) Rapamycin down-regulates phospho-S6. Lymph node cells were harvested from a mouse (no. 1) both before (before tx) and after (after tx) treatment with rapamycin for 3 days. Lymph node cells were also harvested from a mouse treated with rapamycin for 4 weeks (no. 3, long tx) compared with a mouse treated with vehicle for 4 weeks (no. 2, control). Immunoblot of phospho-S6 (Ser 235/236) (top bands), total S6 (middle bands), and β-tubulin (bottom bands) from the mice demonstrates a correlation between biochemical and clinical response to rapamycin in the mice. Lymph cells from treated mice had down-regulation of phospho-S6 compared with lymph cells from untreated mice. (B) Rapamycin down-regulates phospho-Bad. Lymph node cells were harvested from a mouse treated with rapamycin for 4 weeks and a mouse treated with vehicle for 4 weeks. Immunoblot of phospho-Bad (Ser 112; top bands), total Bad (middle bands), and β-tubulin (bottom bands) from the mice demonstrates down regulated phospho-Bad in the rapamycin-treated cells. Results for phospho-Bad (Ser 136) were similar and are not shown. (C) Rapamycin induces caspase-dependent apoptosis in cultured murine ALPS lymphocytes. T cells from lymph node biopsy of CBA-lprcg mice were maintained in culture. Aliquots of 105 lymphocytes were exposed to 100 ng/mL rapamycin for 48 hours and were assessed for apoptosis by flow cytometric staining for annexin V. In addition, prior to treating with rapamycin, aliquots of cells were pretreated for 2 hours with a pancaspase inhibitor, a caspase-9 inhibitor, or a caspase-8 inhibitor. Data represent percentage of apoptotic (annexin V–positive/7-AAD–negative) cells determined by flow cytometric analysis.
Mechanism of action of rapamycin in ALPS. (A) Rapamycin down-regulates phospho-S6. Lymph node cells were harvested from a mouse (no. 1) both before (before tx) and after (after tx) treatment with rapamycin for 3 days. Lymph node cells were also harvested from a mouse treated with rapamycin for 4 weeks (no. 3, long tx) compared with a mouse treated with vehicle for 4 weeks (no. 2, control). Immunoblot of phospho-S6 (Ser 235/236) (top bands), total S6 (middle bands), and β-tubulin (bottom bands) from the mice demonstrates a correlation between biochemical and clinical response to rapamycin in the mice. Lymph cells from treated mice had down-regulation of phospho-S6 compared with lymph cells from untreated mice. (B) Rapamycin down-regulates phospho-Bad. Lymph node cells were harvested from a mouse treated with rapamycin for 4 weeks and a mouse treated with vehicle for 4 weeks. Immunoblot of phospho-Bad (Ser 112; top bands), total Bad (middle bands), and β-tubulin (bottom bands) from the mice demonstrates down regulated phospho-Bad in the rapamycin-treated cells. Results for phospho-Bad (Ser 136) were similar and are not shown. (C) Rapamycin induces caspase-dependent apoptosis in cultured murine ALPS lymphocytes. T cells from lymph node biopsy of CBA-lprcg mice were maintained in culture. Aliquots of 105 lymphocytes were exposed to 100 ng/mL rapamycin for 48 hours and were assessed for apoptosis by flow cytometric staining for annexin V. In addition, prior to treating with rapamycin, aliquots of cells were pretreated for 2 hours with a pancaspase inhibitor, a caspase-9 inhibitor, or a caspase-8 inhibitor. Data represent percentage of apoptotic (annexin V–positive/7-AAD–negative) cells determined by flow cytometric analysis.
Treatment for ALPS patients is challenging. We have demonstrated that rapamycin is potentially an effective medication against lymphoproliferation in ALPS, and studies in humans have demonstrated it is well tolerated.27 Rapamycin causes little nephrotoxicity and neurotoxicity, unlike the immunophilins, but may cause hyperlipidemia and mild myelosuppression.28 We found no overt toxicities in mice treated with rapamycin for 3 months, although this study was designed as an efficacy study and not a long-term murine toxicity study.
Other immunosuppressive agents have been studied in ALPS patients, including cyclosporine (CSA), MMF, and corticosteroids. We compared rapamycin with MMF and found it to be superior in the mice. CSA is a calcineurin inhibitor and decreases proliferation of T lymphocytes. CSA has been shown to have limited efficacy when used alone in ALPS but may be useful in combination with corticosteroids.29,30 MMF inactivates inosine monophosphate, a key enzyme in purine synthesis, resulting in inhibition of proliferating T and B lymphocytes.10,31 MMF was recently demonstrated to be effective in reducing symptoms in a cohort of patients with ALPS; however, MMF is myelosuppressive, inducing neutropenia in as many as 20% of treated patients.32 MMF has also been associated with an increased risk of development of secondary malignancies.33 Patients with ALPS frequently have cytopenias and are already at risk for secondary malignancies, making these potential side effects of MMF worrisome. Corticosteroids induce apoptosis in B and T lymphocytes and are very efficacious in patients with ALPS3,34,35 ; however, long-term treatment with corticosteroids can be extremely toxic. Nevertheless, some patients with ALPS have less severe disease and mild immunosuppressive agents, including MMF, may be effective and safe.
Approximately 10% of patients with ALPS develop secondary malignancies, most commonly leukemia and lymphoma.7 Chronic exposure to immunosuppressive agents bears a theoretical risk for development of secondary malignancies, since the immune system is critical for tumor surveillance. Patients taking immunosuppressive agents, including MMF,7 after solid organ transplantation have been shown to have an increased risk of developing secondary lymphoma. Thus, it is important to use immunosuppressive agents with the least potential to induce malignant transformation in patients with ALPS. In vitro data evaluating immunosuppressive drugs for mutagenic properties in human lymphocyte cultures demonstrated that tacrolimus and MMF are strongly mutagenic even at low doses, CSA is strongly mutagenic but only at high doses, and rapamycin is not mutagenic unless given at very high concentrations and then is only weakly mutagenic.36
A significant percentage of patients with ALPS develop autoimmune manifestations, and treatments are frequently aimed at alleviating symptoms arising as a result of these autoimmune phenomenon. The murine model of ALPS we used for most of these studies, CBA-lprcg, typically develops lymphoproliferation and elevated DNTs, characteristic of ALPS; however, these mice do not develop significant autoimmune manifestations. Studies in children with ALPS have demonstrated a correlation between a decrease in DNTs and lymphoproliferation with improvement in autoimmune manifestations.10,37 MRL-lpr mice are also phenotypically and genotypically similar to humans with ALPS. These mice develop more significant autoantibodies. They also develop glomerulonephritis and vasculitis, characteristics more similar with human systemic lupus erythematosis than ALPS.38 We, therefore, performed most of our experiments in the CBA-lprcg mice, and we used the MRL-lpr mice to assess rapamycin's effect on autoimmunity in the mice. We found rapamycin did reduce autoantibody burden in the MRL-lpr mice. We are cautiously optimistic that the dramatic responses we have demonstrated in the mice may correlate to a reduction in autoimmune manifestations in patients with ALPS.
In summary, we have demonstrated that rapamycin is an effective agent in reducing DNTs, lymphadenopathy, splenomegaly, and autoantibodies in murine ALPS. Based on the results of these data, we plan to initiate a pilot study of rapamycin in children with ALPS that will hopefully lead to a safe, effective, and well-tolerated therapy for this difficult-to-treat disease.
Prepublished online as Blood First Edition Paper, June 6, 2006; DOI 10.1182/blood-2006-01-010124.
Supported by the United States Immunodeficiency Network (USIDNET; grant N01-A1-30070), National Institutes of Health grant K12-CA076931-08, and Partnerships for Cures/Goldman Philanthropic Partnerships and the Rockefeller Brothers Fund (D.T.T., a Charles E. Culpepper Biomedical Pilot Initiative Awardee); and the Sanford Chair and Weinberg Fund (S.A.G.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal