Abstract
We investigated the role of the hematopoietic-specific tetraspanin superfamily member, TSSC6, in platelet function using wild-type mice and TSSC6-deficient mice. TSSC6 is expressed on the surface of murine platelets and is up-regulated by thrombin stimulation, indicating an intracellular pool of TSSC6. Immunoprecipitation/Western blot studies reveal a constitutive physical association of TSSC6 with the integrin αIIbβ3 complex under strong detergent conditions. In vivo evaluation of hemostasis by tail bleeding revealed increased bleeding time, volume of blood lost, and evidence of tail rebleeds in TSSC6 null mice, indicating unstable hemostasis. Using ex vivo techniques, we showed that TSSC6-deficient platelets exhibited impaired kinetics of clot retraction, platelet aggregation at lower doses of PAR-4, and collagen and platelet spreading on fibrinogen in the presence of normal integrin αIIbβ3 expression. TSSC6-deficient platelets showed normal alpha granule secretion, normal “insideout” integrin αIIbβ3 signaling (fluorescein isothiocyanate [FITC]–fibrinogen and JON/A binding), and normal platelet adhesion on fibrinogen. Furthermore, we show that absence of platelet TSSC6 affects the secondary stability of arterial thrombi in vivo upon vascular injury. These data demonstrate that TSSC6 appears to regulate integrin αIIbβ3 “outside-in” signaling events in platelets and is necessary for stability of arterial thrombi in vivo.
Introduction
Tumor-suppressing subchromosomal transferable fragment cDNA 6 (TSSC6), also previously known as Pan hematopoietic expression (Phemx), is a tetraspanin superfamily member (transmembrane 4 superfamily).1,2 TSSC6 contains 4 highly conserved hydrophobic transmembrane domains, 2 extracellular loops, and intracellular N- and C-terminal domains. Its C-terminal cytoplasmic domain is relatively large (33 and 99 amino acids in mouse and human, respectively) compared to other tetraspanin superfamily members. TSSC6 is specifically expressed in hematopoietic organs and tissues where it may play a role in hematopoietic-cell function. Human TSSC6 transcripts have been detected in K562 cells stimulated with phorbol myristate acetate (PMA) to induce differentiation into megakaryocyte lineage.1,3 This finding suggests that TSSC6 protein is likely to be present in platelets and where it may have a functional role.
A hallmark feature of tetraspanin superfamily members is the presence of 4 highly conserved cysteine motifs in the major extracellular domain 2, including CCG, PXSC, and EGC.4 Tetraspanins form physical multimolecular complexes with other transmembrane proteins and transmembrane receptors including integrins that constitute a network termed the tetraspanin web. Several studies have highlighted associations between tetraspanins and integrins, and the best characterized of these include integrin α3β1 with CD151, α6β1 and α6β4 with CD151, and α4β1 with CD81.5 As yet, no biochemical or physical associations of TSSC6 with integrins, including platelet integrin αIIbβ3, have been defined.
There have been several major tetraspanins reported in platelets, including CD9, CD151, TSSC6, and CD63. Of these, CD9 and CD151 are present on the platelet surface and are also associated with the inner surface of alpha granules, along with their molecular partner, integrin αIIbβ3 and its soluble ligand fibrinogen.6 In contrast, CD63 is present on the membrane of dense granules and lysosomes and is relocated to the platelet surface following platelet activation and exocytosis.7 CD63 has been demonstrated to be physically associated with a multimolecular complex of CD9: integrin αIIbβ3 on the surface of activated platelets.8 The intracellular localization of TSSC6 in platelets is presently unknown.
In platelets, CD9 has been functionally linked with the low-affinity IgG receptor, FcγRIIa,9,10 although others suggest that CD9 may regulate platelet function independently of FcγRIIa.11 CD151 functions independently of platelet FcγRIIa and regulates the “outside-in” signaling properties of integrin αIIbβ3 in murine platelets that are devoid of FcγRIIa.12,13 Recently, CD63 was reported to modulate human platelet spreading on immobilized fibrinogen in an integrin αIIbβ3-dependent manner.7 These studies demonstrate that the formation of tetraspanin multimolecular complexes with integrin αIIbβ3 in platelets is of functional importance.
In this study, we report that the hematopoietic-specific tetraspanin superfamily member, TSSC6, is expressed both on the cell surface and in intracellular pools of murine platelets. In this context, TSSC6 is physically associated with the integrin αIIbβ3 complex in platelets and has a direct role in regulating “outside-in” integrin αIIbβ3-mediated signaling in murine platelets. In addition, TSSC6 functions as a molecular facilitator to stabilize platelet aggregates in high-shear thrombosis following vascular injury in vivo.
Materials and methods
Mice
TSSC6-deficient mice were generated in the laboratory of our authors (L.R., M.D.W.) and maintained on a C57BL/6 background.14 Four- to 8-week-old wild-type (WT) and TSSC6 null mice were used in this study. Experimental procedures were approved by the Austin Health Animal Ethics Committee.
Antibodies (Ab's) and FITC-fibrinogen
Anti–mouse integrin β3 mAb, FITC-conjugated anti–mouse P-selectin mAb, FITC-conjugated anti–mouse CD3 mAb, and phycoerythrin (PE)–conjugated antirat Ab were purchased from BD Pharmingen (San Diego, CA). Anti–human integrin complex–specific αIIbβ3 Ab, P2, was obtained from Beckman Coulter (Gladesville, New South Wales). FITC-conjugated anti–mouse CD44 mAb was obtained from Beckman Coulter (Brea, CA), while rat anti–mouse CD9 mAb and JON/A-PE mAb were from Emfret Analytics (Wurzburg, Germany). Anti–mouse TSSC6 monoclonal antibodies (15G3 and 14A6) were generated by immunizing Wistar rats with FDC-P1 and DP16 cell lines. Primary screening of hybridoma supernatants were performed by flow cytometry of a mixture of COS-7 cells engineered to express the FLAG epitope in EC1 (COS-7 TSSC6/EC1 FLAG) and parental COS-7 cells.15 Selected supernatants were rescreened against COS-7 TSSC6/EC1 FLAG and panel of murine hematopoietic-cell lines. Polyclonal anti–human TSSC6 (Phemx) Ab was obtained from Abcam (Cambridge, United Kingdom). FITC-conjugated fibrinogen was generated by incubating 20 mg human fibrinogen with 5 mg FITC (Sigma Chemical, St Louis, MO) in 0.15 M carbonate buffer pH 9.0 for 1 hour at room temperature. Labeled fibrinogen was then purified by separation over a PD10 column (Amersham Pharmacia, Piscataway, NJ).
Preparation of washed platelets
Eight hundred microliters of whole blood was collected by cardiac puncture of the inferior vena cava of each mouse under halothane anesthesia. The whole blood was anticoagulated in 3.8% (wt/vol) trisodium citrate in a ratio of 1 part anticoagulant to 9 parts blood. For washed platelet assays, 50 ng/mL prostaglandin E1 (PGE1) (Sigma Chemical) was included to minimize platelet activation. Platelets were obtained from platelet-rich plasma and washed in Ringers citrate dextrose buffer (RCD) pH 6.5. Platelet counts were normalized between wild-type and TSSC6 null platelets before functional assays were performed.
Hemostatic parameters
Platelet aggregation
Platelet aggregation was stimulated by 2.5 to 20 μg/mL collagen, 2.5 to 10 μM adenosine diphosphate (ADP), 5 to 20 μg/mL calcium ionophore, 125 to 500 μM protease-activated receptor (PAR-4) agonist peptide, 2.5 to 20 μg/mL collagen-related peptide (CRP), and monitored in a 4-channel Chronolog aggregometer (Chrono-log, Havertown, PA). Fibrinogen was added at a final concentration of 100 μg/mL.
Flow cytometry analysis
Washed platelets were resuspended in RCD buffer pH 6.5 and stimulated with a range of agonists as indicated. Platelets (100 × 109/L) were incubated with either FITC-conjugated rat anti–mouse P-selectin (10 μg/mL), FITC-conjugated human fibrinogen, or PE-conjugated JON/A antibody for 1 hour at 37°C. Samples were washed in RCD pH 6.5 containing 0.2% (wt/vol) bovine serum albumin and then analyzed immediately. For quantitative flow cytometry, QuantiBrite PE beads at 4 different levels of molecules/bead were used to derive a calibration curve (Becton Dickinson, San Jose, CA). For all measurements, a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA) was used.
Platelet adhesion and spreading
Platelet adhesion to fibrinogen was performed as previously described.13 Briefly, coverslips were coated with 100 μg/mL fibrinogen, and washed platelets (100 × 109/L) were added. Attached platelets were visualized using differential interference contrast (DIC) microscopy (×63 DIC oil objective), and the numbers of attached platelets per high-power field were counted. Similarly, for platelet-spreading experiments, unlabeled platelets were visualized using DIC microscopy (×63 DIC oil objective). Spread platelets were differentiated from nonspread platelets counted per high-power field according to the criteria of Goodman.17 Using this criteria, spreading platelets (S) were defined as a hyaloplasm spread between pseudopodia or fully spread platelets (FS) as hyaloplasm extensively spread with no distinct pseudopodia. As mouse platelets undergo a more limited degree of spreading on fibrinogen than human platelets, the S forms of platelets were more common than FS.
In vivo thrombosis model
Wild-type or TSSC6-deficient C57BL/6 mice (4-5 weeks old) were anesthetized with a ketamine/xyzaline (200:10 mg/kg) mixture, and the mesentery was exteriorized through a midline abdominal incision. A catheter was inserted into the jugular vein to deliver rhodamine dye and anesthetic as required. Mesenteric arterioles (60-100 μm) were visualized with a Zeiss Axiovert 135 microscope (Zeiss, Oberkochen, Germany), captured with a Dage MTI camera (Dage MTI, Michigan City, IN), and video recorded using Streamline software using a Dell computer. A filter paper strip of 1 × 4 mm was dipped in a 462 mM (7.5% wt/vol) FeCl3 solution for 3 seconds and then applied to a 2- to 5-mm length of arteriole for 4 minutes and then removed.18 Vessels were monitored for 30 minutes after injury or until full occlusion (blood flow stopped). Parameters assessed were (1) the time required for initial thrombus formation of diameter more than 20 μm; (2) the number of thrombi of diameter more than 20 μm; (3) clot occlusion time of vessel that involves the time for the blood to stop flowing; (4) the increase in area and diameter over 1 minute for forming thrombi more than 20 μm; (5) the time the blood clot was stable in the vessel (seconds); (6) duration of instability of blood clot in occluded vessel over time (seconds) (if observed); and (7) the number of emboli observed after vessel has clotted (termed secondary stability).
Platelet count determination
Washed platelets were diluted using the Unopette microcollection system according to the manufacturer's instructions (Becton Dickinson, Franklin Lakes, NJ). Platelet counts were performed using a hemocytometer.
Statistical analysis
Data are presented as mean ± SEM. Statistical significance was assessed by unpaired Student t test and the Mann-Whitney test as indicated using GraphPad Prism statistical software version 4 (GraphPad, San Diego, CA). P values less than .05 were considered significant.
TSSC6 is expressed on the surface and in an intracellular pool of platelets in complex with integrin αIIbβ3. (A-B) Flow cytometric analysis of TSSC6 surface expression on resting and thrombin-stimulated (0-5 U/mL) wild-type murine platelets. P-selectin exposure on thrombin-stimulated (1 U/mL) wild-type murine platelets in the absence and presence of FITC-antimouse P-selectin mAb is included as a positive control marker of alpha granule release. Platelets were stained with the indicated anti–murine 14A6 TSSC6 monoclonal antibody followed by a secondary FITC-conjugated antirat antibody. Data were collected through a live platelet gate based on forward versus side scatter profiles on a FACS Calibur flow cytometer. Results are cumulative data derived from 3 independent experiments and presented as mean fluorescence intensity (MFI) ± SEM (n = 3). (C) 1.5 mg of human platelet lysates solubilized with indicated detergents were immunoprecipitated with antibodies directed against normal mouse IgG1, PECAM-1, GPIb-IX-V complex, and integrin complex-specific αIIbβ3 Ab, P2. Samples were resolved on 12.5% SDS-PAGE under reducing conditions and Western blotted for TSSC6 with a polyclonal anti–human TSSC6 antibody.
TSSC6 is expressed on the surface and in an intracellular pool of platelets in complex with integrin αIIbβ3. (A-B) Flow cytometric analysis of TSSC6 surface expression on resting and thrombin-stimulated (0-5 U/mL) wild-type murine platelets. P-selectin exposure on thrombin-stimulated (1 U/mL) wild-type murine platelets in the absence and presence of FITC-antimouse P-selectin mAb is included as a positive control marker of alpha granule release. Platelets were stained with the indicated anti–murine 14A6 TSSC6 monoclonal antibody followed by a secondary FITC-conjugated antirat antibody. Data were collected through a live platelet gate based on forward versus side scatter profiles on a FACS Calibur flow cytometer. Results are cumulative data derived from 3 independent experiments and presented as mean fluorescence intensity (MFI) ± SEM (n = 3). (C) 1.5 mg of human platelet lysates solubilized with indicated detergents were immunoprecipitated with antibodies directed against normal mouse IgG1, PECAM-1, GPIb-IX-V complex, and integrin complex-specific αIIbβ3 Ab, P2. Samples were resolved on 12.5% SDS-PAGE under reducing conditions and Western blotted for TSSC6 with a polyclonal anti–human TSSC6 antibody.
Results
TSSC6 is expressed on the surface and in intracellular pools of murine platelets
Reverse transcriptase–polymerase chain reaction studies of TSSC6 transcripts in tissues and cells have demonstrated that TSSC6 is a hematopoietic-specific tetraspanin.1 To determine the presence of TSSC6 protein expressed on the surface of resting platelets and to determine if this expression is up-regulated by intracellular granule release, we examined the binding of specific monoclonal anti–murine TSSC6 antibodies (14A6) to either resting or thrombin-stimulated murine platelets, monitored by flow cytometry. As shown in Figure 1A, a 2-fold increase in fluorescence intensity was observed in resting murine platelets using specific monoclonal antibodies directed against TSSC6 (n = 3). This was further increased another 2-fold by thrombin stimulation under conditions of alpha granule release, as shown by P-selectin exposure (Figure 1B). This increase in TSSC6 expression plateaued around 2 U/mL thrombin (n = 3). As a negative control, TSSC6 knockout platelets were included and showed minimal background with the TSSC6 monoclonal antibody (Figure 1B). In addition, using quantitative flow cytometry to determine relative molecules per cell for TSSC6 versus CD9 on murine platelets, we determined that CD9 was expressed 5-fold higher in expression than TSSC6 on murine platelets (8930 ± 1218 molecules/cell versus 2281 ± 214 molecules/cell). These results indicate that TSSC6 is expressed on the surface of resting murine platelets and is found in an intracellular pool in murine platelets.
TSSC6–/– mice display features of unstable hemostasis in vivo. Hemostasis in 6- to 8-week-old mice was assessed using an in vivo tail-bleeding assay. (A) Time taken for cessation of bleeding for wild-type and TSSC6–/– mice (n = 20). P < .05 (Student t test). (B) Volume of blood lost during tail-bleeding time for wild-type and TSSC6–/– mice (n = 20). P < .05 (Student t test). (C) Rebleeding occurrences in wild-type and TSSC6–/– mice (n = 20). P < .005 (Student t test). Errors bars indicate SEM.
TSSC6–/– mice display features of unstable hemostasis in vivo. Hemostasis in 6- to 8-week-old mice was assessed using an in vivo tail-bleeding assay. (A) Time taken for cessation of bleeding for wild-type and TSSC6–/– mice (n = 20). P < .05 (Student t test). (B) Volume of blood lost during tail-bleeding time for wild-type and TSSC6–/– mice (n = 20). P < .05 (Student t test). (C) Rebleeding occurrences in wild-type and TSSC6–/– mice (n = 20). P < .005 (Student t test). Errors bars indicate SEM.
TSSC6 is physically complexed to integrin αIIbβ3 in platelets
To investigate a physical association of integrin αIIbβ3 complex with TSSC6 in human platelets, immunoprecipitation studies were performed using antibodies directed against isotype control mAb, normal mouse IgG1, platelet glycoprotein platelet-endothelial-cell adhesion molecule-1 (PECAM-1), GPIb-IX-V complex, and integrin αIIbβ3 complex–specific mAb, P2, in the presence of either 1% (vol/vol) Triton X-100 or with 1% (vol/vol) Brij-96, 1% (vol/vol) Thesit, and 1% (vol/vol) CHAPS 3-([(3-cholamidopropyl)dimethylamonio]-1-propyl sulfonate). Following immunoprecipitation, protein complexes were resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and the presence of TSSC6 detected by Western blotting. As shown in Figure 1C, TSSC6 was detectable in integrin αIIbβ3 (P2) immunoprecipitates, but not normal mouse IgG1, GPIb-IX-V complex, and PECAM-1 immunoprecipitates, under all detergent conditions including 1% (vol/vol) Triton X-100, 1% (vol/vol) Brij-96, 1% (vol/vol) Thesit, and 1% (vol/vol) CHAPS. These results are consistent with a level 1 direct interaction.4
TSSC6 knockout mice display features of unstable hemostasis in vivo
As TSSC6 is expressed by platelets but not vascular endothelium,1,3 we wanted to test the effect of deletion of a hematopoietic-specific tetraspanin in an in vivo tail-bleeding assay. As shown in Figure 2, there was a small but significant (P < .05; n = 20) difference in the mean tail-bleeding times for wild-type versus TSSC6 knockout mice. Consistent with this feature, the mean volume of blood lost during the tail-bleeding time was 2-fold greater for TSSC6 knockout mice compared to wild-type mice (P < .05; n = 20). In addition, the percentage of rebleeds for TSSC6 knockout mice was significantly 4-fold higher (38% versus 9%; P < .005; n = 20) compared to wild-type mice, indicating unstable hemostasis. This is not attributed to any alteration in platelet count, as TSSC6 knockout mice have comparable platelet counts as wild-type mice.14 Based upon this in vivo bleeding defect, the TSSC6 knockout mice appear to have an underlying platelet functional defect.
Absence of platelet TSSC6 results in secondary instability in platelet thrombi formed in vivo. (A) Thrombus formation in vivo was monitored on mesenteric arterioles after topical application in 7.5% FeCl3. Time to artery congestion and primary clot formation in seconds after injury. Each symbol represents 1 monitored arteriole for each wild-type versus TSSC6–/– mouse. Wild-type: 773.4 ± 40.2 seconds; TSSC6–/–: 885.7 ± 36.3 seconds. P < .05; n = 30. (B) Time of clot stability in seconds after injury and occlusion of vessel. Each symbol represents 1 monitored arteriole for each wild-type versus TSSC6–/– mouse. Wild type: 314.4 ± 47.9 seconds; TSSC6–/–: 186.7 ± 38.4 seconds. P < .05; n = 11. (C) Number of thrombi embolizing more than 20 μm in diameter formed during the 30- to 45-minute observation period. Each symbol represents 1 monitored arteriole for each wild-type versus TSSC6–/– mouse. Wild-type: 4.0 ± 1.3 emboli; TSSC6–/–: 9.0 ± 1.9 emboli. P < .05; n = 23.
Absence of platelet TSSC6 results in secondary instability in platelet thrombi formed in vivo. (A) Thrombus formation in vivo was monitored on mesenteric arterioles after topical application in 7.5% FeCl3. Time to artery congestion and primary clot formation in seconds after injury. Each symbol represents 1 monitored arteriole for each wild-type versus TSSC6–/– mouse. Wild-type: 773.4 ± 40.2 seconds; TSSC6–/–: 885.7 ± 36.3 seconds. P < .05; n = 30. (B) Time of clot stability in seconds after injury and occlusion of vessel. Each symbol represents 1 monitored arteriole for each wild-type versus TSSC6–/– mouse. Wild type: 314.4 ± 47.9 seconds; TSSC6–/–: 186.7 ± 38.4 seconds. P < .05; n = 11. (C) Number of thrombi embolizing more than 20 μm in diameter formed during the 30- to 45-minute observation period. Each symbol represents 1 monitored arteriole for each wild-type versus TSSC6–/– mouse. Wild-type: 4.0 ± 1.3 emboli; TSSC6–/–: 9.0 ± 1.9 emboli. P < .05; n = 23.
Platelet TSSC6 is required for stable platelet thrombus formation upon FeCl3-induced vascular injury
To determine the consequences of TSSC6 deficiency in vivo, we tested wild-type and TSSC6–/– mice in a model of lethal pulmonary thromboembolism induced by infusion of type 1 fibrillar collagen (data not shown). All wild-type and TSSC6–/– mice died within 240 seconds, with more than 60% reduction in circulating platelet counts within 3 minutes of challenge (data not shown). Consistent with these observations, histologic sections of lung tissue and analysis of occlusive thrombi in the lungs reveals similar numbers of occluded vessels between wild-type and TSSC6–/– mice (data not shown).
We studied the effect of TSSC6 deficiency on thrombus formation in wild-type and TSSC6–/– mice using a well-characterized mouse model of arterial injury. The ferric chloride (FeCl3) oxidative injury model was used to induce formation of free radicals to disrupt the vascular endothelium in mesenteric arterioles.18 Following application of FeCl3 to the mesentery, platelets in both wild-type and TSSC6–/– mice rapidly commenced interacting with the injured vessel wall and within 10 to 15 minutes after the injury, vessel occlusion occurred. However, the clotted vessels in TSSC6–/– mice were less stable over time, with microaggregates and thrombi breaking loose from the site of injury or from platelet aggregates. Typically, TSSC6–/– mice showed a slight delay in time to stable clot formation and reduced stability of clotted vessel formed in vivo following thrombus occlusion over time (Figure 3A-C) (P < .05). In contrast, TSSC6–/– mice showed no abnormality in the time to first thrombi more than 20 μm, number of thrombi more than 20 μm, the increase in area or diameter over 1 minute for forming thrombi more than 20 μm (P > .05; n = 25) (data not shown). Collectively, these data demonstrate that TSSC6 is required for the secondary stabilization of platelet-rich thrombi in FeCl3-injured mesenteric arterioles.
Impaired “outside-in” integrin αIIbβ3-mediated signaling in TSSC6–/– platelets
Based upon in vivo evidence that platelet TSSC6 contributes to the stabilization of platelet thrombus formation, we wanted to investigate the possibility that TSSC6 may modulate the function of integrin αIIbβ3 in murine platelets. As the formation of stable platelet aggregates is dependent upon the association of activated integrin αIIbβ3 in complex with its soluble ligand, fibrinogen, we examined integrin αIIbβ3-dependent ex vivo platelet responses. This was achieved by studying kinetics of clot retraction, platelet spreading, and platelet aggregation. As shown in Figure 4A, a delay in the kinetics of clot retraction was observed in TSSC6 knockout platelets compared with wild-type platelets. Wild-type platelets started retracting at 20 minutes and were completely retracted by 1 hour, while TSSC6 knockout platelets showed only partial retraction by 60 minutes. This defect was not attributable to reduced expression of integrin αIIbβ3 on the surface of TSSC6 knockout platelets, as demonstrated by flow cytometry (Figure 4B). In addition, CD44 and CD9 expression were normal on TSSC6 knockout platelets. These results indicate that the hematopoietic-specific tetraspanin TSSC6 regulates integrin αIIbβ3 signaling in murine platelets.
Delayed kinetics of clot retraction for TSSC6–/– platelets in the presence of normal integrin αIIbβ3 expression. (A) Photographs showing in vitro kinetics of clot retraction over a 21-hour time frame using platelet rich plasma (PRP) (normalized platelet counts) from wild-type and TSSC6-deficient mice. Samples were treated with 2.5 units thrombin. Each photograph is representative of at least 3 experiments. (B) The expression of surface markers on platelets was determined by staining with an isotype control (FITC-CD3), positive control FITC-CD44 mAb, FITC-CD9, and FITC-integrin β3 mAb for both wild-type and TSSC6–/– platelets. FITC-labeled samples were analyzed on a FACS Calibur analyzer. Results are cumulative data derived from 3 independent experiments and presented as MFI ± SEM.
Delayed kinetics of clot retraction for TSSC6–/– platelets in the presence of normal integrin αIIbβ3 expression. (A) Photographs showing in vitro kinetics of clot retraction over a 21-hour time frame using platelet rich plasma (PRP) (normalized platelet counts) from wild-type and TSSC6-deficient mice. Samples were treated with 2.5 units thrombin. Each photograph is representative of at least 3 experiments. (B) The expression of surface markers on platelets was determined by staining with an isotype control (FITC-CD3), positive control FITC-CD44 mAb, FITC-CD9, and FITC-integrin β3 mAb for both wild-type and TSSC6–/– platelets. FITC-labeled samples were analyzed on a FACS Calibur analyzer. Results are cumulative data derived from 3 independent experiments and presented as MFI ± SEM.
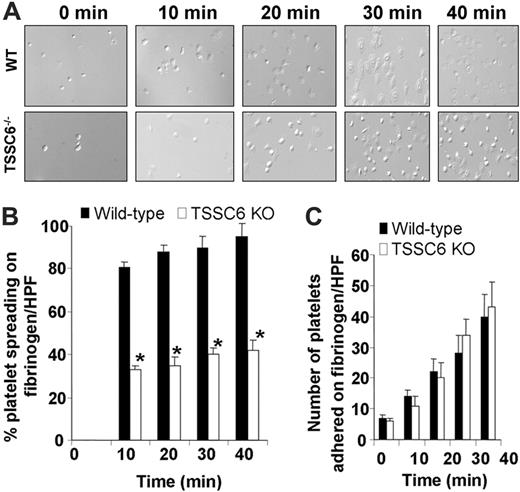
TSSC6–/– platelets display restricted cytoskeletal reorganization upon spreading on immobilized fibrinogen. (A) Washed wild-type and TSSC6–/– platelets were allowed to adhere for 0 to 40 minutes at 37°C to a fibrinogen matrix. Adherent platelets were fixed and imaged by DIC microscopy. Images were captured with an Axiovert 135 microscope (Zeiss, Oberkochen, Germany) with a 63×/1.25 oil immersion lens and a PixeLINK megapixel firewire camera (model PL-A661; PixeLINK, Ottawa, ON, Canada) and PixeLINK software version 3.2. (B) The percentage of spread platelets for each genotype were quantitated per high-powered field (*P < .05, n = 3). DIC images shown are a representative of 3 independent experiments. (C) The number of adherent platelets for each genotype was quantitated per high-powered field (P > .05; n = 3). Results are representative of 3 independent experiments.
TSSC6–/– platelets display restricted cytoskeletal reorganization upon spreading on immobilized fibrinogen. (A) Washed wild-type and TSSC6–/– platelets were allowed to adhere for 0 to 40 minutes at 37°C to a fibrinogen matrix. Adherent platelets were fixed and imaged by DIC microscopy. Images were captured with an Axiovert 135 microscope (Zeiss, Oberkochen, Germany) with a 63×/1.25 oil immersion lens and a PixeLINK megapixel firewire camera (model PL-A661; PixeLINK, Ottawa, ON, Canada) and PixeLINK software version 3.2. (B) The percentage of spread platelets for each genotype were quantitated per high-powered field (*P < .05, n = 3). DIC images shown are a representative of 3 independent experiments. (C) The number of adherent platelets for each genotype was quantitated per high-powered field (P > .05; n = 3). Results are representative of 3 independent experiments.
To test if the “outside-in” signaling properties of integrin αIIbβ3 are defective in TSCC6 knockout platelets, we next examined the formation of filopodia and spreading on fibrinogen over time using differential interference contrast (DIC) microscopy. As shown in Figure 5A-B, TSSC6–/– platelets exhibited a significant 3-fold reduction in their capacity to spread on immobilized fibrinogen over time compared to wild-type platelets (*P < .05; n = 3). Under DIC microscopy, a larger proportion of TSSC6–/– platelets adhered to immobilized fibrinogen typically showed rounded morphology with limited filopodia and spreading (Figure 5A-B). This defect is related to integrin αIIbβ3-dependent platelet spreading and not platelet adhesion, as an equivalent number of platelets were observed to adhere to immobilized fibrinogen over time for both wild-type and TSSC6–/– platelets (P > .05; n = 3) (Figure 5C). These results indicate that TSSC6–/– platelets have restricted cytoskeletal reorganization when adhered to immobilized fibrinogen.
TSSC6–/– platelets display normal platelet aggregation responses to PAR-4, ADP, type 1 collagen, and calcium ionophore. Aggregation responses of PRP (platelet count adjusted to 100 × 109/L) for wild-type and TSSC6–/– mice were determined following activation with different concentrations of various agonists: PAR-4 agonist peptide (125-250 μM), ADP (5-20 μM), type 1 collagen (5-20 μg/mL), and calcium ionophore (2.5-10 μg/mL), respectively.
TSSC6–/– platelets display normal platelet aggregation responses to PAR-4, ADP, type 1 collagen, and calcium ionophore. Aggregation responses of PRP (platelet count adjusted to 100 × 109/L) for wild-type and TSSC6–/– mice were determined following activation with different concentrations of various agonists: PAR-4 agonist peptide (125-250 μM), ADP (5-20 μM), type 1 collagen (5-20 μg/mL), and calcium ionophore (2.5-10 μg/mL), respectively.
As integrin αIIbβ3 is required in platelet aggregation, we next examined the platelet aggregation responses of TSSC6 knockout platelets compared with wild-type platelets using a range of platelet agonists. As shown in Figure 6, wild-type and TSSC6 knockout platelets display equivalent platelet aggregation profiles at higher concentrations of PAR-4 (250 μM), calcium ionophore (2.5-10 μg/mL), type 1 acid-soluble collagen (10-20 μg/mL), and ADP (5-20 μM). In contrast, at lower concentrations of PAR-4 (125-150 μM) and type 1 collagen (5 μg/mL), TSSC6 knockout platelets showed reduced platelet aggregation responses and, in some cases, evidence of disaggregation in the secondary phase of platelet aggregation. This defect in TSSC6 knockout platelets also was observed with low-dose thrombin (≤ 0.25 U/mL) in a washed platelet system (data not shown). Therefore, based upon these results, TSSC6 knockout platelets display normal amplitude and slope of platelet aggregation responses at higher concentrations of PAR-4, ADP, calcium ionophore, and type 1 collagen. However, TSSC6 knockout platelets display reduced amplitude and slope of platelet aggregation responses at lower concentrations of PAR-4 and type 1 collagen.
Normal “inside-out” integrin αIIbβ3-mediated signaling in TSSC6–/– platelets
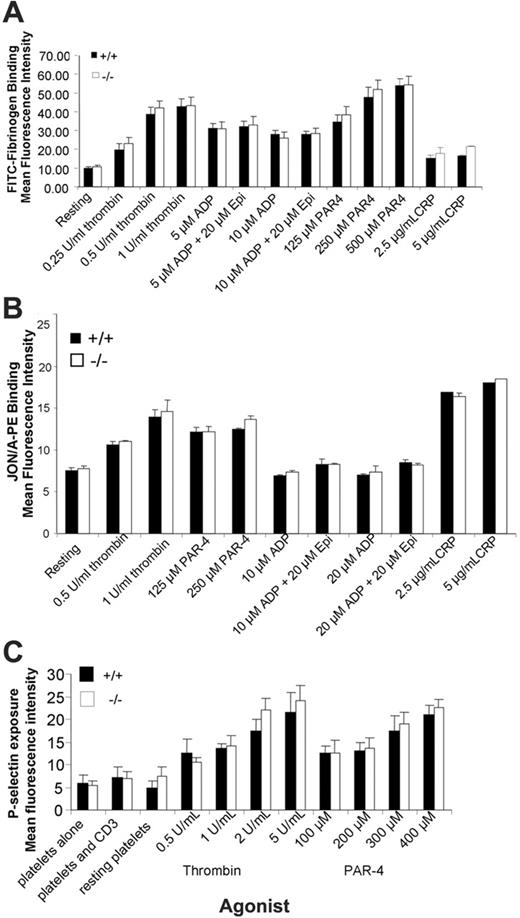
As TSSC6–/– platelets primarily show a defect in “outside-in” integrin αIIbβ3-mediated signaling, we wanted to confirm that TSSC6–/– platelets have normal “inside-out” integrin αIIbβ3-mediated signaling properties. To test for a defect in integrin αIIbβ3 activation, the ability of wild-type and TSCC6–/– platelets to bind FITC-fibrinogen and JON/A mAb under resting conditions and following CRP (2.5-5 μg/mL), thrombin (0.5-1 U/mL), PMA (20 μM), ADP (10-20 μM), and ADP (10-20 μM) + epinephrine (20 μM). The binding of soluble FITC-fibrinogen or JON/A mAb was assessed by flow cytometry. As shown in Figure 7A-B, agonist-induced stimulation of wild-type and TSSC6–/– platelets demonstrated a similar ability to bind soluble FITC-fibrinogen as well as JON/A mAb, which recognizes the active conformation of murine integrin αIIbβ3. In addition, we wanted to examine the kinetics of alpha granule release of TSSC6 knockout platelets. As shown in Figure 7C, the surface expression of P-selectin on thrombin and PAR-4 stimulated platelets derived from wild-type and TSSC6 knockout platelets were equivalent over a range of thrombin (0.5-5 U/mL) and PAR-4 (100-400 μM) concentrations. Therefore, based upon these results, it would appear that TSSC6–/– platelets have normal “inside-out” integrin αIIbβ3-mediated signaling properties and normal alpha granule release.
TSSC6–/– platelets display normal soluble FITC-fibrinogen binding, JON/A mAb binding, and alpha granule release following treatment with thrombin, PAR-4 peptide, ADP, and ADP in synergy with epinephrine. (A) FACS analysis of FITC-conjugated fibrinogen binding to platelets stimulated with thrombin (1 U/mL), PAR-4 (250 μM), PMA (20 μM), ADP (10 μM), ADP (10 μM) + epinephrine (20 μM), or unstimulated (control). Results are cumulative data from 3 independent assays and are presented as MFI ± SEM. (B) Flow cytometric analysis of JON/A-PE mAb binding to platelets stimulated with 0.5 to 1.0 U/mL thrombin, 125 to 250 μM PAR-4, 10 to 20 μM ADP, 10 to 20 μM ADP + 20 μM epinephrine, 2.5 to 5 μg/mL CRP, or unstimulated (control). Results are cumulative data from 3 independent experiments and are presented as MFI ± SEM. (C) Surface expression of P-selectin was determined for washed platelets stimulated by 0.5 to 5 U/mL thrombin or 100 to 400 μM PAR-4 agonist peptide and then stained with either a buffer control and FITC–P-selectin mAb for wild-type and TSSC6–/– platelets. FITC-labeled samples were analyzed on a FACS Calibur analysis. Results are cumulative data from 3 independent experiments and are presented as MFI ± SEM.
TSSC6–/– platelets display normal soluble FITC-fibrinogen binding, JON/A mAb binding, and alpha granule release following treatment with thrombin, PAR-4 peptide, ADP, and ADP in synergy with epinephrine. (A) FACS analysis of FITC-conjugated fibrinogen binding to platelets stimulated with thrombin (1 U/mL), PAR-4 (250 μM), PMA (20 μM), ADP (10 μM), ADP (10 μM) + epinephrine (20 μM), or unstimulated (control). Results are cumulative data from 3 independent assays and are presented as MFI ± SEM. (B) Flow cytometric analysis of JON/A-PE mAb binding to platelets stimulated with 0.5 to 1.0 U/mL thrombin, 125 to 250 μM PAR-4, 10 to 20 μM ADP, 10 to 20 μM ADP + 20 μM epinephrine, 2.5 to 5 μg/mL CRP, or unstimulated (control). Results are cumulative data from 3 independent experiments and are presented as MFI ± SEM. (C) Surface expression of P-selectin was determined for washed platelets stimulated by 0.5 to 5 U/mL thrombin or 100 to 400 μM PAR-4 agonist peptide and then stained with either a buffer control and FITC–P-selectin mAb for wild-type and TSSC6–/– platelets. FITC-labeled samples were analyzed on a FACS Calibur analysis. Results are cumulative data from 3 independent experiments and are presented as MFI ± SEM.
Discussion
An emerging theme in tetraspanin research is the concept that tetraspanins associate with each other and with transmembrane receptors to form tetraspanin webs. This concept is supported by recent studies in tetraspanin knockout mice where CD37–/–, CD151–/–, CD81–/–, and TSSC6–/– mice showing similar phenotypes of hyperproliferative T cells14,16,19,20 and CD9–/– and CD81–/– mice displayed similar phenotypes of sperm-egg fusion fertility defects.21-24 In addition, the “outside-in” integrin αIIbβ3 signaling defect and unstable hemostasis phenotype of CD151–/– mice13 is somewhat similar to the phenotype of TSSC6–/– mice reported in this study.
TSSC6 appears to be crucial in the negative regulation of peripheral T-lymphocyte proliferation. TSSC6–/– T cells demonstrated enhanced responses to concanavalin A, anti-CD3 alone, and anti-CD3 + anti-CD28. This heightened responsiveness by TSSC6–/– T cells was attributed to increased interleukin 2 (IL-2) production following T-cell antigen receptor stimulation.14 However, its functional role in hematopoietic cells is undefined. A recent report suggested that TSSC6 may be expressed by platelets based upon detection of TSSC6 mRNA transcript in K562 cells differentiated into megakaryocytic lineage.1,3 In this study, we provide evidence for the presence of TSSC6 expression both on the surface as well as within intracellular pools of platelets. Furthermore, TSSC6 was found to be constitutively associated in a physical complex with the major platelet glycoprotein, integrin αIIbβ3 in resting platelets (Figure 1). From a functional perspective, TSSC6–/– mice displayed evidence of unstable hemostasis in vivo (Figure 2), and TSSC6–/– platelets demonstrated features of an “outside-in” integrin αIIbβ3 signaling defect in vitro (Figures 4, 5, 6, 7). Finally, TSSC6 was found to play a role in thrombosis based upon instability in platelet thrombi formed in a murine model of FeCl3-induced in vivo vascular injury (Figure 3A-C).
Previous studies have demonstrated that in normal resting platelets, tetraspanin superfamily members are located in different subcellular compartments. CD9 is highly expressed on the platelet surface and is located in alpha granules within the cytoplasms of platelets together with integrin αIIbβ3, fibrinogen, and von Willebrand factor (VWF).6 In contrast, CD63 is located on the membranes of dense granules and lysosomes.7 In both cases, platelet activation resulted in relocation of these tetraspanin superfamily members to the plasma membrane, where they form a complex with integrin αIIbβ3. Based upon our current study, TSSC6 surface expression on platelets was up-regulated by stimulation with thrombin, indicating release of TSSC6 from an intracellular pool. This observation that TSSC6 and integrin αIIbβ3 are stored in the same intracellular granular pool and migrate to the plasma membrane after platelet stimulation lends support to the concept of a close constitutive association between TSSC6 and integrin αIIbβ3. Whether TSSC6 interacts directly or indirectly with integrin αIIbβ3 is not known, but it is likely that the relationship is complicated, potentially involving other tetraspanin multimolecular complexes. The exact number of copies of TSSC6 per platelet has not been determined; however, based upon quantitative fluorescence-activated cell sorting scanner (FACS) data, it would appear to be less than integrin αIIbβ3, CD9, and CD151, which are expressed at a higher density (magnitude of expression: integrin αIIbβ3 > CD9 > CD151 > TSSC6).
The fact that tetraspanin superfamily members can form lateral associations with integrins suggests that TSSC6 plays a role as an organizer of multimolecular complexes involving integrin αIIbβ3 to regulate its adhesive/signaling events. Following this, our studies revealed that TSSC6–/– mice displayed features of unstable hemostasis as shown by a tendency for rebleeds, as well as increased blood loss and tail-bleeding times in vivo. In addition, TSSC6–/– platelets have an “outside-in” integrin αIIbβ3 signaling defect shown by a delay in kinetics of clot retraction and restricted cytoskeletal reorganization in platelet spreading on fibrinogen. Many of these features resemble the phenotypes observed in the tetraspanin superfamily member CD151–/– mice13 and the integrin αIIbβ3 Y747759F mice.25 In addition, recent studies using antibody cross-linking of CD63 suggests that CD63 also may be functionally linked with integrin αIIbβ3 in platelets.7
In the in vivo injury model, TSSC6–/– mice exhibited secondary instability in formed platelet thrombi, resulting in frequent embolization compared with wild-type mice. As TSSC6 is restricted to hematopoietic lineages, it would suggest that platelet TSSC6 is required for maintaining stable platelet thrombus formation in vivo. It would appear that TSSC6 contributes to the formation of stable integrin αIIbβ3-mediated platelet aggregates within a thrombus and particularly in the anchorage of platelet aggregates to the injured vessel wall interface. In these experiments, cohorts of large platelet aggregates were observed to be detaching from the entire thrombus as well as the injured vessel wall interface under high shear flow conditions. Based upon these observations, it is most likely that TSSC6 plays a key role at high shear in platelet aggregation rather than fibrin formation that stabilizes the growing thrombus.
The embolization observed in the absence of TSSC6 may have important clinical implications since fatal pulmonary embolus formation has been reported in afibrinogenemia patients.26,27 At this stage, no naturally occurring TSSC6-deficient patients have been reported to test the possibility that spontaneous bleeding problems are encountered due to the reduced inability to form stable platelet plugs at site(s) of injury.
Several studies have highlighted that tetraspanins do not modulate integrin-dependent static cell adhesion, but regulate integrin adhesion strengthening.28 Tetraspanin CD151 was found to play a key role in regulating the adhesion strengthening of the integrin α6β1, particularly involving the C-terminal region of CD151.29 In addition, the CD81 tetraspanin was found to facilitate the adhesion strengthening of vascular-cell adhesion molecule 1 (VCAM-1) under shear flow conditions.30 Taken together, these studies suggested that tetraspanins may exert their effect by regulation of the “outside-in” properties of integrin-mediated signaling.
This study places into context the functional importance of a hematopoietic-specific tetraspanin, TSSC6, in stabilizing arterial thrombi but not thrombus growth in vivo involving a functional relationship with integrin αIIbβ3 “outside-in” signaling in platelets. While mechanisms of early events in platelet activation are well known, many of the late events that control platelet thrombus growth and stability are still poorly defined. One of the key unanswered questions raised by Brass and colleagues is which mechanisms prevent destabilization of the hemostatic plug and clot retraction during the time needed for wound healing to occur.31 Several molecules have been implicated in contact-facilitated events in platelets that regulate thrombus growth and stability, including platelet-derived CD40L and Eph kinases/ephrins, that also regulate integrin αIIbβ3 “outside-in” signaling events.32,33 Specifically, CD40L–/– mice showed a delay in platelet thrombus growth and blood vessel occlusion following vascular injury combined with a decrease in thrombus stability.32 These previous studies suggested that CD40L behaves as an integrin αIIbβ3 ligand that is necessary for stability of arterial thrombi. Based upon the results of our current study, the hematopoietic-specific tetraspanin TSSC6 appears to be a new integrin αIIbβ3 partner that modulates its “outside-in” signaling properties necessary for stability but not growth of arterial thrombi in vivo. This TSSC6:integrin αIIbβ3 interaction in platelets is therefore likely to be an important mechanism for stabilization of the hemostatic plug required to stop bleeding and subsequent clot retraction that is required during the process of wound healing.
Prepublished online as Blood First Edition Paper, May 23, 2006; DOI 10.1182/blood-2006-02-004267.
Supported by grants from the National Heart Foundation of Australia and the National Health and Medical Research Council of Australia (D.E.J.). D.E.J. is a recipient of a National Health and Medical Research Council (NHMRC) Senior Research Fellowship. L.R. is a recipient of an NHMRC Principal Research Fellowship. M.J.H. is a recipient of an NHMRC R. Douglas Wright Fellowship.
M.W.G. and L.-M.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
L.-M. Lau is a recipient of a National Heart Foundation Biomedical PhD Scholarship and is a postgraduate student of the University of Melbourne.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal