Abstract
Thrombin exerts pleiotropic effects on endothelial cells, including the release of microparticles (EMPs) that disseminate and exchange information with vascular cells. Nevertheless, the mechanisms leading to their generation are not elucidated. We performed microarray analysis to identify genes involved in EMP release by the endothelial cell line HMEC-1 in response to thrombin. We identified a group of genes linked to the cytoskeleton reorganization family. Among these, the Rho-kinase ROCK-II presented a high transcription rate. ROCK-I, another Rho-kinase isoform, was not modulated by thrombin. Pharmacologic inhibition of Rho-kinases or specific depletion of ROCK-II by short interfering (si) RNA inhibited thrombin-induced EMP release. In contrast, ROCK-I mRNA silencing did not modify EMP generation by thrombin. Exposure of HMEC-1 to thrombin in presence of the caspase-2 selective inhibitor Z-VDVAD-FMK prevented ROCK-II cleavage and inhibited the thrombin-induced EMP release. These events were observed in absence of cell death. Our data clearly identified ROCK-II as a target of thrombin in EMP generation. They indicated that the 2 Rho-kinases did not share identical functions. The involvement of caspase-2 in ROCK-II activation independently of cell death points out a novel signaling pathway that emphasizes the proteolytic activity of caspase in EMP generation in response to cell activation.
Introduction
Thrombin is a serine protease playing a central role in the coagulation cascade and hemostasis. Generated at sites of vascular injury in the vicinity of a thrombus, thrombin promotes platelet activation, adhesion, and trafficking of inflammatory cells into sites of injury.1 Moreover, thrombin exerts pleiotropic effects on the endothelium controlling the proliferative/reparative responses to injury and promoting both proinflammatory and procoagulant endothelial phenotypes.2 Thrombin activities are mediated by the activation of the G-protein–linked protease-activated receptors (PARs), mainly PAR-1 on the endothelial cells.3 Recently, the gene profiling of primary endothelial cells in response to thrombin indicates changes in the transcription of a multitude of genes,2,4 which results in modifications of the endothelial cell shape, cytoskeleton rearrangement, and increased permeability.5 Despite the large body of experiments describing the effects of thrombin on the endothelium, little is known on its capacity to generate endothelial cell microparticles6 and the mechanisms involved in their release have never been reported.
Microparticles found in the extracellular space derive from membrane blebs. Blebbing is a reversible dynamic event that occurs during cell activation or apoptosis.7 Viable cells display plasma membrane blebbing when spreading, migrating, dividing, or under conditions of stress. Blebbing depends on intracellular forces generated by the actin-myosin cytoskeleton and is driven through the activation of actin-myosin contraction8,9 by the small GTP-binding protein Rho and its major effectors, the Rho-kinases I and II,10 which in turn are cleaved in an active form by caspase-dependent or -independent pathways.11-13 This cleavage leads to the subsequent phosphorylation of downstream substrates and cell morphologic changes.14 Membrane blebbing ultimately leads to cellular fragmentation with, as consequence, the release of shedded microparticles into the extracellular space. Cellular fragmentation does not occur when blebbing is prevented by addition of the rho kinase inhibitor Y27632,15,16 supporting of a role of ROCK in microparticle generation.
All the cells can release microparticles. The interest for microparticles has substantially increased because of their ability to act at distant sites as well as locally, and to propagate the functional state of their parent cell.17-20 In response to apoptotic stimuli or activation, endothelial cells release endothelial microparticles (EMPs)21 that exert paracrine effects on cells of the vascular system22 or autocrine actions on endothelial cells themselves.23
In this work, we first establish that thrombin initiates EMP generation from the human microvascular endothelial cell line HMEC-1. We then identify the molecular mechanisms controlling this generation. Microarray analysis reveals an up-regulated transcription of genes linked to cytoskeleton arrangement. Among this functional family, ROCK-II shows the highest transcription rate. We demonstrate that ROCK-II, and not ROCK-I, is a major regulator of the thrombin-induced EMP release. We show for the first time that, despite an absence of cell death, caspase-2 plays a role in EMP release by controlling the proteolytic activation of ROCK-II. Taken together, these data point out a novel signaling cascade leading to EMP generation that occurs during cell activation.
Materials and methods
Endothelial cell culture
The human microvascular endothelial cell line-1 (HMEC-1) was grown at subconfluence24 and used between passages 8 and 12-HMEC-1 cells were serum-starved overnight and stimulated with human thrombin (Sigma, St Louis, MO) at different doses and times, or with the PAR agonist peptide SFLLRN (20 μM; Euromedex, Mundolsheim, France), or with PPACK-thrombin (0.1 μM; Invitrogen, Carlsbad, CA), the inactive form of thrombin. Viability of HMEC-1 cells in all the experimental conditions was determined by the incorporation of the fluorescent dye Alamar Blue (Molecular Probes, Eugene, OR)
Endothelial microparticle detection
One milliliter from cell-conditioned media (CCM) of unstimulated or thrombin-stimulated HMEC-1 cells (7 × 105 cells on 12-well plates) were collected and cleared from cell debris by centrifugation at 5000g for 5 minutes. EMPs were quantified by flow cytometry in CCM.20 Aliquots of 10 μL CCM were labeled by phosphatidylserine probing using annexin V–FITC kit (Beckman Coulter, Fullerton, CA). For counting, a known amount of fluorescent latex beads (Flowcount; Beckman Coulter) was added as internal standard to samples before flow cytometry analysis. Samples were analyzed on a Coulter Epics XL according to their size and fluorescence. Using 1.0 μm latex beads as gating parameters, EMPs were defined as particles less than or equal to 1 μm in size. EMPs were enumerated from the gate corresponding to annexin V–FITC+ events. Number of EMPs were calculated using the formula: EMP = Num × Vt/Vf × Vs, where Num is the number of EMPs passed through the flow cytometer, Vt is the total volume of conditioned media, Vf is the volume speed, and Vs is the counted sample volume. Results were expressed as the number of EMPs/μL of supernatant.
HMEC-1 transfections
HMEC-1 cells were seeded in 12-well plates and transfected at 60% confluence by magnetofection (OZ Biosciences, Marseille, France) as described by the manufacturers.
For studies with RhoA, HMEC-1 cells were transfected with 2.5 μg cDNA plasmids corresponding to the wild-type (pRK5-mycRhoA; WT) and the dominant-negative (pRK5-mycRhoDN19; ΔDN19) forms of RhoA and the empty vector pRK5 (Pharmingen BD Biosciences, Franklin Lakes, NJ) using Polymag II magnetofection beads. After 48 hours, HMEC-1 cells were kept untreated or stimulated with 1 IU/mL thrombin for 18 hours.
For studies of RNA interference, transfections were performed with ROCK-I– and ROCK-II–validated short interfering (si) RNA duplexes (50 nM) or a scrambled nonsilencing siRNA as negative control (Santa Cruz Biotechnology, Santa Cruz, CA) complexed with Silence MAG beads (OZ Biosciences) as described by the manufacturers. At 48 hours after siRNA transfection, cells were harvested to determine the level of ROCK-I and ROCK-II proteins by immunoblotting, or were stimulated with 1 IU/mL thrombin for 18 hours.
ROCK detection and activity
Cell lysates (25 μg) from thrombin-stimulated HMEC-1 or unstimulated cells were separated on 5% to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. After transfer, immunoblotting was performed with rabbit anti–ROCK-II or monoclonal anti–ROCK-I antibodies (Santa Cruz Biotechnology). Immunoblots exposed to secondary peroxidase–conjugated goat antirabbit (Fab′)2 antibody (Jackson Laboratories, Bar Harbor, ME) were visualized with enhanced chemiluminescence (ECL) reagent. Equal protein loading was ensured by reblotting with anti–human α-tubulin.
Rho-kinase activity was determined in cell lysates by a nonisotopic Rho-kinase assay kit (Cyclex, Nagano, Japan) as indicated by the manufacturer. Briefly, the assay is based on the capacity of the Rho-kinases present in the cell lysates to phosphorylate, on threonine residue, their substrate MBS. The phosphorylation is then detected by addition of a peroxidase-coupled monoclonal antibody specific for phospho-MBS and the chromogenic substrate tetra-methylbenzidine.
Immunofluorescence microscopy
HMEC-1 cells were cultured to subconfluence on 8 chamber glass slides (BD Falcon, Bedford, MA). After treatment, they were fixed in 4% formaldehyde in phosphate-buffered saline (PBS) and permeabilized with 0.1% Triton X100. Actin cytoskeleton was stained with Phalloidin Texas red (Molecular Probes). Localization of vinculin was performed by indirect immunofluorescence. Cells were incubated with 1/400th diluted monoclonal antivinculin antibody (clone hVIN-1; Sigma), followed by a sequential incubation with a 1/100th diluted fluorescein (FITC)–labeled goat-antimouse antibody (Jackson Laboratories). Fluorescence images were acquired on a Nikon Eclipse TE2000-U video-imaging inverted microscope using a ×40 objective magnification, and were captured using the Lucias software for Nikon.
cDNA arrays
Custom high-density cDNA arrays were designed as previously described.25 Briefly, Nylon filters (Hybond-N+ 2 × 7cm2 membranes; Amersham, Uppsala, Sweden) contained 8074 spotted polymerase chain reaction (PCR) products from 7874 selected IMAGE human cDNA clones (MRC Rosalind Franklin Centre for Genomics Research; Geneservice, Cambridge, United Kingdom) and 200 control clones. The IMAGE clones were divided into 6664 cDNA and 1210 expressed sequence tags (ESTs). About 10% of the genes included in this clone set are represented by 2 or more different cDNA clones, providing internal controls to assess the reproducibility of gene expression measurements.
RNA extraction and cDNA labeling
Three independent experiments were performed using HMEC-1 cells stimulated with thrombin (1 IU/mL) for 0.5, 1, 2, 4, 6, 12, and 18 hours. Total RNA was isolated using the RNeasy mini kit (Qiagen, Valencia, CA). The RNA concentration was spectrophotometrically estimated and RNA integrity was checked by a microfluidic RNA 6000 Nano-Assay using the 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Reverse transcription was performed as previously described, using 2 μg total RNA in the presence of α33P-dCTP. A large excess of oligo(dT) primers was added during cDNA synthesis, and the labeled probe was annealed with poly(dA)80 to ensure the complete saturation of poly(A) tails. Hybridizations were performed for 48 hours at 68°C.25,26 Hybridizations were performed using 3 different extracts on 3 different cDNA arrays. After washing, arrays were exposed to phosphor imaging plates, which were scanned using a BAS 5000 (Fuji, Tokyo, Japan) at a 25-μm resolution.
Data processing and analysis
After image acquisition, the data were processed using the ArrayGauge software (Fuji, Tokyo, Japan). Spots with overestimated intensities due to neighborhood effects were manually excluded. For each array, background intensity was calculated on the basis of the average of the 400 lowest values and subtracted as described.27 For each sample, the 6000 highest values were flagged.
Expression profiling analysis. Data were filtered (100%), log2 transformed, and centered relative to the median for each gene and each array. Hierarchical supervised clustering in the array axis was applied to the data set using Cluster software and results were visualized with Treeview software.28
Differential analysis. Distinct classes of samples, each of them containing the 3 replicates of the same time, were defined. Only clones present in all the samples and exhibiting a standard deviation (SD) less than or equal to 0.35 in each class were kept. The mean of the normalized values of each gene from thrombin-stimulated HMEC-1 cells was divided by the mean of normalized values of the same gene in unstimulated cells. Genes with a ratio of 2.0 or above were considered positively regulated by thrombin, whereas those that had a ratio of 0.5 or below were considered negatively regulated.
All data are compliant with Minimum Information About a Microarray Experiment (MIAME) guidelines and have been submitted to Gene Expression Omnibus (GEO) database29 (accession no. E-MEXP-477).
Genes of interest were followed up by referring to the web SOURCE and Gene Ontology (http://www.geneontology.org) databases for functional information and subsequently categorized into functional classes. Data are shown in Table S1, available on the Blood website (see the Supplemental Materials link at the top of the online article).
Quantitative real-time PCR
Two-step real-time (RT)–PCR was performed. Total RNA from the same sample as those used for the microarray experiments was reversed-transcribed using the High Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA). cDNA product (200 ng) was amplified in a 20-μL reaction on ABI Prism 7700 sequence detection system (Applied Biosystems) using the Taqman Universal PCR Master Mix. Primer pairs and Taqman probes were purchased as predesigned and validated reagents by the manufacturers (Assays-on-Demand Gene Expression Probes; Applied Biosytems). The PCR program consisted of an initial denaturation at 95°C for 10 minutes followed by amplification for 40 cycles (95°C for 15 seconds, 60°C for 1 minute, and 72°C for 15 seconds). A standard curve for serial dilutions of 18S rRNA used as calibrator was similarly generated. Data were generated from each reaction, analyzed using ABI Prism software, and normalized to 18S RNA. Quantification was performed using the ΔΔCTcalculation. The relative standard curve method was used to calculate the amplification difference between thrombin-stimulated and unstimulated cells.
Cell death detection and caspase inhibition
HMEC-1 cells were cultured in 96-well plates for 18 hours in the presence or absence of thrombin (1 IU/mL). Apoptosis was quantitatively determined by the assay of histone-associated DNA fragments nucleosomes in the cytoplasm. Cells were trypsinized and an equal number between all the samples were lysed. Apoptosis was quantified in the lysates using the Cell Death ELISA kit (Roche Diagnostics, Basel, Switzerland) as described by the manufacturer. The stimulation of HMEC-1 cells by the combination of human TNF-α (25 ng/mL) and cyloheximide (2.5 μg/mL) (TNF/CHX) was used as a positive control of HMEC-1 apoptosis.
When required, HMEC-1 cells were preincubated 1 hour with the selective inhibitors of ROCK Y-27632 (1 μM) and HA1077 (10 μM) or with 2 μM total or selective caspase inhibitors (Biovision, Mountain View, CA) and stimulated with thrombin (1 IU/mL) in the presence of the inhibitors.
Caspase-2 activity assay
The activity of caspase-2 was assayed in cell lysates from thrombin-stimulated or unstimulated control cells using the caspase-2 fluorimetric assay kit (Biovision) as indicated by the manufacturer. Briefly, cell lysates were incubated with VDVAD-AFC, the fluorescent substrate of caspase-2. Fluorescence was determined in a plaque-reader fluorimeter (Cytofluor 4000; PerSeptive Biosystems, Framingham, MA) using excitation and emission wavelengths of 400 nm and 505 nm, respectively.
Statistical analysis
Values presented are means plus or minus the standard error of the mean (SEM). Data were compared with the use of a 2-tailed unpaired Student t test, using P values below .5 as the level of significance.
Results
Endothelial microparticle release by thrombin
The ability of thrombin to induce generation of EMPs in cell-conditioned medium of the endothelial cell line HMEC-1 was first studied by flow cytometry using annexin V binding to phosphatidylserine exposed on EMPs. Flow cytometric data representative of 4 independent experiments, indicated that thrombin enhanced EMP release in CCM compared with untreated control cells (Figure 1A). EMP release increased between 0.1 IU/mL and 1.0 IU/mL and then reached a plateau (Figure 1B; P < .001 vs control). The short agonist peptide SFLLRN (TRAP; 20 μM) for the protease-activated receptor PAR-1 was as effective as thrombin (1 IU/mL) on EMP release (Figure 1B; not significant vs thrombin-stimulated HMEC-1 cells). Conversely, PPACK-thrombin (0.1 μM), which lacks enzymatic activity, did not mimic the effect of thrombin on EMP release (Figure 1B; P < .001 vs thrombin-stimulated HMEC-1 cells). The level of EMP in response to thrombin was about half of that observed in response to TNF-α used as positive control (Figure 1A-B). The release of EMPs in CCM was time dependent (Figure 1C; P < .001 at 18 hours vs control cells at the same time) with a significant increase between 6 and 18 hours. Accordingly, the following experiments were performed with an 18-hour stimulation with 1 IU/mL thrombin.
Gene profiling expression in response to thrombin
To gain insight into the targets involved in EMP release by thrombin, time-induced changes in the gene expression profile of HMEC-1 cells in response to 1 IU/mL thrombin was studied by using custom-made cDNA arrays consisting in 9862 human cDNA. RNA was isolated from untreated or thrombin-stimulated HMEC-1 cells after 0.5, 1, 2, 4, 6, 12, and 18 hours of stimulation. Analysis by hierarchical clustering of the arrays identified clusters of genes up- or down-regulated at the different times of experiments (Figure S1, Table S1). A maximum of genes was transcribed between 1 and 2 hours after the onset of thrombin stimulation. To analyze genes with the most fluctuating expression levels across the samples, we then performed a differential analysis between data from stimulated versus untreated cells. Among them, 157 genes showed an up-regulation of their expression and 31 presented a down-regulated expression (Table S1) in response to thrombin. Many of these genes were related to cell growth, proliferation, receptors, intracellular signaling, and cell organization. In this latter class, changes in the expression were observed for genes linked to the cytoskeleton reorganization (Table S1). ROCK-II, one of the 2 Rho-associated coiled-coil–containing serine threonine kinase (ROCK) isoforms,30 was highly expressed in this family. ROCKs are downstream effectors of Rho-GTPases, and activate several downstream targets implicated in distinct cellular functions. Data from microarray analysis indicated an increased gene expression of some of these targets (Table S1, in bold). Figure 2A showed the time-dependent increase in gene profiling linked to the Rho-GTPase pathway. Among them, ROCK-II was the most expressed.
Dose and time-dependent release of endothelial microparticles (EMP) by thrombin. (A) Flow cytometric detection and quantification of EMP. Conditioned cell culture media (CCM) were recovered from serum-starved HMEC-1 untreated (control) or stimulated for 18 hours with thrombin (1 IU/mL) or TNF-α (100ng/mL). Size-selected events 1μm were plotted according to fluorescence for specific annexin V–FITC binding (FL1) on FL1/side scatter histograms (top row). Positive labeled events are included in gate D and considered as annexin V–positive EMP (bottom row). Representative count of EMP versus fluorescence intensity. These plots are representative of 4 independent experiments. (B) Effect of thrombin, PAR-1 agonist TRAP, inactive thrombin-PPACK and TNF-α on EMP release by HMEC-1. Serum-starved HMEC-1 cells were kept unstimulated (control) or stimulated for 18 hours with increasing doses of thrombin and EMP were assayed in CCM, as described in “Materials and methods.” The bar graph represented the mean value of EMP per 1 × 106 HMEC-1 cells ± SEM from 10 independent experiments. (C) Time response curve of thrombin-induced EMP release. EMPs were recovered from CCM from serum-starved HMEC-1 cells kept untreated (▴) or stimulated for different times with 1 IU/mL thrombin (•). The curves represent the mean values of EMP per 1 × 106 cells ± SEM from 6 independent experiments. *P < .01, ***P < .001, for thrombin-stimulated versus unstimulated (control) HMEC-1 cells. NS indicates not significant for thrombin-versus TRAP-stimulated HMEC-1 cells.
Dose and time-dependent release of endothelial microparticles (EMP) by thrombin. (A) Flow cytometric detection and quantification of EMP. Conditioned cell culture media (CCM) were recovered from serum-starved HMEC-1 untreated (control) or stimulated for 18 hours with thrombin (1 IU/mL) or TNF-α (100ng/mL). Size-selected events 1μm were plotted according to fluorescence for specific annexin V–FITC binding (FL1) on FL1/side scatter histograms (top row). Positive labeled events are included in gate D and considered as annexin V–positive EMP (bottom row). Representative count of EMP versus fluorescence intensity. These plots are representative of 4 independent experiments. (B) Effect of thrombin, PAR-1 agonist TRAP, inactive thrombin-PPACK and TNF-α on EMP release by HMEC-1. Serum-starved HMEC-1 cells were kept unstimulated (control) or stimulated for 18 hours with increasing doses of thrombin and EMP were assayed in CCM, as described in “Materials and methods.” The bar graph represented the mean value of EMP per 1 × 106 HMEC-1 cells ± SEM from 10 independent experiments. (C) Time response curve of thrombin-induced EMP release. EMPs were recovered from CCM from serum-starved HMEC-1 cells kept untreated (▴) or stimulated for different times with 1 IU/mL thrombin (•). The curves represent the mean values of EMP per 1 × 106 cells ± SEM from 6 independent experiments. *P < .01, ***P < .001, for thrombin-stimulated versus unstimulated (control) HMEC-1 cells. NS indicates not significant for thrombin-versus TRAP-stimulated HMEC-1 cells.
Thrombin-induced increase in ROCK-II transcription and protein in HMEC-1 cells. (A) Time-dependent changes in the expression levels of genes linked to Rho pathways. cDNA microarrays were performed as described in “Materials and methods.” The graph represents individual profiling of the genes described in Table S1. (B) Validation of microarray data with real-time quantitative PCR analysis of ROCK mRNA: changes in ROCK-II mRNA (▴) and ROCK-I mRNA (•) were normalized to that observed with 18S ribosomal mRNA, as described in “Materials and methods.” Results were analyzed by the ΔΔCt method that reflects the difference in threshold for the target gene relative to that of 18S in each sample, and are expressed as fold induction of ROCK-II or ROCK-I mRNA expression in thrombin-stimulated HMEC-1 cells compared with time-matched untreated HMEC-1 cells in 3 independent experiments. (C) Representative experiments of time-dependent modulation in ROCK-II and ROCK-I proteins in thrombin-treated HMEC-1 cells. Each cell lysate (20 μg) was resolved on 4% to 12% SDS-PAGE gradient under reducing conditions. The blots were probed with anti–ROCK-II monoclonal antibody (top panel) or anti–ROCK-I polyclonal rabbit antibody (center panel) or with antitubulin as loading controls (bottom panel). (D) Cytoskeleton rearrangement in response to thrombin. Serum-starved HMEC-1 cells were cultured on glass chambers and stained for actin or vinculin, as described in “Materials and methods.” After 4 hours of stimulation, images were acquired on an inversed fluorescent microscope and were captured using the Lucias software for Nikon. Control (i, ii, v, vi) indicates unstimulated cells; thrombin (iii, iv, vii, viii), HMEC-1 cells stimulated with 1 IU/mL thrombin for 4 hours. When required, 1μM Y27632 was added 1 hour before stimulation and was maintained in the culture medium throughout the stimulation step. Objective magnification, 40×/0.60 NA.
Thrombin-induced increase in ROCK-II transcription and protein in HMEC-1 cells. (A) Time-dependent changes in the expression levels of genes linked to Rho pathways. cDNA microarrays were performed as described in “Materials and methods.” The graph represents individual profiling of the genes described in Table S1. (B) Validation of microarray data with real-time quantitative PCR analysis of ROCK mRNA: changes in ROCK-II mRNA (▴) and ROCK-I mRNA (•) were normalized to that observed with 18S ribosomal mRNA, as described in “Materials and methods.” Results were analyzed by the ΔΔCt method that reflects the difference in threshold for the target gene relative to that of 18S in each sample, and are expressed as fold induction of ROCK-II or ROCK-I mRNA expression in thrombin-stimulated HMEC-1 cells compared with time-matched untreated HMEC-1 cells in 3 independent experiments. (C) Representative experiments of time-dependent modulation in ROCK-II and ROCK-I proteins in thrombin-treated HMEC-1 cells. Each cell lysate (20 μg) was resolved on 4% to 12% SDS-PAGE gradient under reducing conditions. The blots were probed with anti–ROCK-II monoclonal antibody (top panel) or anti–ROCK-I polyclonal rabbit antibody (center panel) or with antitubulin as loading controls (bottom panel). (D) Cytoskeleton rearrangement in response to thrombin. Serum-starved HMEC-1 cells were cultured on glass chambers and stained for actin or vinculin, as described in “Materials and methods.” After 4 hours of stimulation, images were acquired on an inversed fluorescent microscope and were captured using the Lucias software for Nikon. Control (i, ii, v, vi) indicates unstimulated cells; thrombin (iii, iv, vii, viii), HMEC-1 cells stimulated with 1 IU/mL thrombin for 4 hours. When required, 1μM Y27632 was added 1 hour before stimulation and was maintained in the culture medium throughout the stimulation step. Objective magnification, 40×/0.60 NA.
Identification of ROCK-II as a target of thrombin
The up-regulation of ROCK-II observed with the cDNA microarray was then confirmed by real-time quantitative PCR (qRT-PCR). ROCK-II mRNA was highly up-regulated 4 hours after thrombin (1 IU/mL) stimulation (Figure 2B). Immunoblotting of cell lysates from thrombin-stimulated HMEC-1 cells indicated a time-dependent increase in the approximately 160 kDa uncleaved ROCK-II protein. In addition, a cleaved, approximately 130-kDa form accumulated up to 4 hours and then gradually disappeared (Figure 2C, upper panel). Interestingly, thrombin did not modify the expression of ROCK-I, the other isoform of ROCK (Figure 2B), or its protein expression (Figure 2C, central panel). Moreover, thrombin did not promote any cleavage of the molecule. Rho-kinases are involved in cytoskeleton rearrangement. We examined changes in actin and vinculin staining in thrombin-treated HMEC-1 cells (Figure 2D). In control unstimulated cells, actin fibers were rearranged into a fine reticular cortical network (Figure 2Di). Immunofluorescent localization of vinculin which identifies focal adhesion, showed a fine diffuse pattern (Figure 2Dii). A 4-hour stimulation of HMEC-1 monolayers with thrombin induced a dissolution of the dense peripheral actin band with the fine reticular cortical F-actin network replaced by thicker stress fibers (Figure 2Diii), and vinculin appeared as dense, brightly staining clumps found at areas that corresponded to focal adhesion (Figure 2Div). Treatment of HMEC-1 cells with Y27632 (1 μM), a selective inhibitor of Rho-kinases, prevented the morphologic changes induced by thrombin either on actin or on vinculin (Figure 2Dvii-viii) without displaying any effect on control cells (Figure 2Dv-vi). These data indicated that ROCK-II was a target of thrombin in HMEC-1 cells. Its high gene expression level and its cleavage in response to thrombin led us to study its involvement in thrombin-induced EMP release.
Involvement of ROCK-II in thrombin-induced EMP release
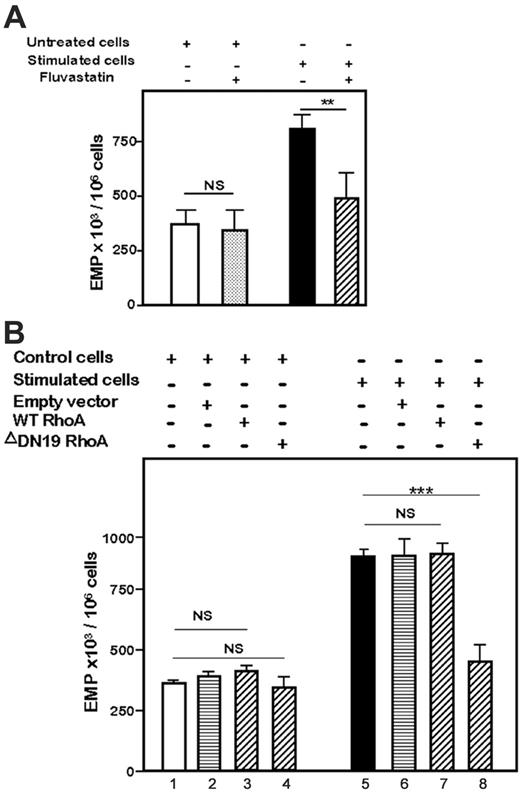
RhoA and its downstream target ROCK both control membrane traffic31 and bleb formation.7,32 So, we first investigated RhoA involvement in thrombin-induced EMP generation. Preincubation and of HMEC-1 cells with the 3-hydroxy-3-methylglutaryl (HMG)–CoA reductase inhibitor fluvastatin 0.01 μM known to block the Rho/Rho-kinase signaling pathway and culture of the cells with 1 IU/mL thrombin in the presence of the drug, prevented EMP release by thrombin (Figure 3A; P < .001 vs thrombin-stimulated cells) and had no effect on the basal EMP release. Transfection of HMEC-1 cells with WT or defective-inactive ΔDN19 plasmids confirmed the involvement of RhoA in the EMP release (Figure 3B). In response to thrombin, EMP release was equivalent in untransfected HMEC-1 cells and in WT RhoA-transfected HMEC-1 cells (Figure 3, lane 7 vs lane 5; not significant). Transfection of ΔDN19 RhoA plasmid markedly reduced thrombin-induced EMP release (Figure 3, lane 8 vs lane 5; P < .001 vs thrombin-stimulated cells). The empty vector displayed no effect on both the thrombin-stimulated EMP level (Figure 3, lane 6 vs lane 5) and the basal level (Figure 3, lane 2 vs lane 1). These data indicated that RhoA mediates thrombin-induced EMP release.
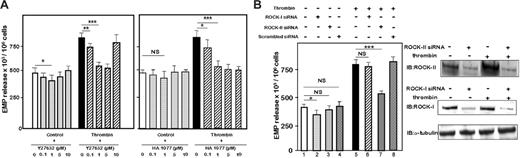
The role of ROCK in EMP generation was then investigated. Preincubation of HMEC-1 cells with increasing doses of the selective inhibitors of ROCK Y-27632 (Figure 4A, left panel) or HA-1077 (Figure 4A, right panel)15 and stimulation with 1 IU/mL thrombin in presence of the drugs greatly inhibited EMP release, indicating that ROCK contributed to the thrombin-induced EMP generation. In addition, Y-27632 partially but not significantly inhibited the basal level of EMP in untreated cells.
Involvement of Rho-GTPases in EMP release by thrombin. (A) Effect of fluvastatin on EMP release by 1 IU/mL thrombin. Serum-starved HMEC-1 cells were preincubated for 1 hour with fluvastatin (0.01 μM), washed, and then kept untreated (control) or stimulated for 18 hours with thrombin in presence of identical drug concentrations. (B) Effect of RhoA transfections on EMP level. HMEC-1 cells were either untransfected or transiently transfected with wild type RhoA (WT), inactive ΔDN19RhoA, or empty vector, as described in “Materials and methods.” Two days later, the cells were kept untreated or were stimulated with 1 IU/mL thrombin for the next 18 hours. At the end of the 18-hour stimulation, EMPs were quantified in CCM, as described in “Materials and methods.” Bar graphs in panels A and B show the mean values ± SEM for 3 independent experiments. **P < .01, ***P < .001; NS indicates not significant.
Involvement of Rho-GTPases in EMP release by thrombin. (A) Effect of fluvastatin on EMP release by 1 IU/mL thrombin. Serum-starved HMEC-1 cells were preincubated for 1 hour with fluvastatin (0.01 μM), washed, and then kept untreated (control) or stimulated for 18 hours with thrombin in presence of identical drug concentrations. (B) Effect of RhoA transfections on EMP level. HMEC-1 cells were either untransfected or transiently transfected with wild type RhoA (WT), inactive ΔDN19RhoA, or empty vector, as described in “Materials and methods.” Two days later, the cells were kept untreated or were stimulated with 1 IU/mL thrombin for the next 18 hours. At the end of the 18-hour stimulation, EMPs were quantified in CCM, as described in “Materials and methods.” Bar graphs in panels A and B show the mean values ± SEM for 3 independent experiments. **P < .01, ***P < .001; NS indicates not significant.
We hypothesized that ROCK-II plays an essential role in EMP release. We used siRNAs to target ROCK-II and ROCK-I, and a scrambled siRNA as negative control. The efficiency of the transfection was monitored by immunoblotting 48 hours after transfection. A net decrease in protein expression was observed in HMEC-1 cells transfected with the specific siRNA (Figure 4B, right panel). The ROCK-II–targeted siRNA significantly inhibited the release of EMPs from thrombin-treated HMEC-1 cells (Figure 4B, left panel, lane 7 vs lane 5; P < .001). The inhibition driven by ROCK-II siRNA was completely thrombin dependent. Indeed, in the absence of thrombin, ROCK-II siRNA had no visible effect on the basal level of EMPs (Figure 4B, lane 3 vs lane 1; not significant). ROCK-I–targeted siRNA did not significantly modify the levels of EMP release in response to thrombin stimulation (Figure 4B, lane 6 vs lane 5; not significant). It should be noted that ROCK-I siRNA slightly inhibited the basal release of EMPs (Figure 4B, lane 2 vs lane 1). The scrambled siRNA did not modify the basal (Figure 4B, lane 4 vs lane 1) or the thrombin-stimulated EMP levels (Figure 4B, lane 8 vs lane 5). All together, these data showed that axis RhoA/ROCK is involved in thrombin-mediated EMP generation, and that ROCK-II but not ROCK-I played a central role.
Requirement of ROCK-II but not ROCK-I for thrombin-induced EMP release. (A) Inhibition of EMP release by Rho-kinase inhibitors. Serum-starved HMEC-1 were preincubated 1 hour with different concentrations of Rho-kinase inhibitor Y27632 (left panel) or HA1077 (right panel) and then kept untreated or stimulated for 18 hours with 1 IU/mL thrombin in presence of the same quantities of inhibitors. EMPs were quantified in the cell-conditioned medium, as described in “Materials and methods.” Bar graphs represented mean values ± SEM for 5 independent experiments. *P < 0.5, **P < .01, ***P < .001. NS indicates not significant; control, unstimulated cells. (B) ROCK-II mediated EMP release by thrombin. Forty-eight hours after transfection with ROCK-II or ROCK-I or scrambled siRNA(s), HMEC-1 cells were kept untreated or were stimulated with 1 IU/mL thrombin for 18 hours, and EMPs were assayed in conditioned cell culture medium as above (right panel). Bar graphs represented mean values ± SEM for 3 independent experiments. *P < .05, ***P < .001; NS indicates not significant. Changes in the expression of ROCK-II and ROCK-I proteins were monitored by immunoblot 48 hours after transfection of HMEC-1 cells with ROCK-II or ROCK-I siRNA(s) (left panel).
Requirement of ROCK-II but not ROCK-I for thrombin-induced EMP release. (A) Inhibition of EMP release by Rho-kinase inhibitors. Serum-starved HMEC-1 were preincubated 1 hour with different concentrations of Rho-kinase inhibitor Y27632 (left panel) or HA1077 (right panel) and then kept untreated or stimulated for 18 hours with 1 IU/mL thrombin in presence of the same quantities of inhibitors. EMPs were quantified in the cell-conditioned medium, as described in “Materials and methods.” Bar graphs represented mean values ± SEM for 5 independent experiments. *P < 0.5, **P < .01, ***P < .001. NS indicates not significant; control, unstimulated cells. (B) ROCK-II mediated EMP release by thrombin. Forty-eight hours after transfection with ROCK-II or ROCK-I or scrambled siRNA(s), HMEC-1 cells were kept untreated or were stimulated with 1 IU/mL thrombin for 18 hours, and EMPs were assayed in conditioned cell culture medium as above (right panel). Bar graphs represented mean values ± SEM for 3 independent experiments. *P < .05, ***P < .001; NS indicates not significant. Changes in the expression of ROCK-II and ROCK-I proteins were monitored by immunoblot 48 hours after transfection of HMEC-1 cells with ROCK-II or ROCK-I siRNA(s) (left panel).
Involvement of caspase-2 in EMP release and ROCK-II cleavage
It is well established that caspases mediated membrane blebbing. We determined whether caspases controlled the thrombin-induced EMP generation. Preincubation and stimulation of HMEC-1 cells in the presence of z-VAD-FMK (2 μM), a pan-caspase inhibitor, was sufficient to abrogate the EMP release induced by thrombin (Figure 5A, lane 10 vs lane 9; P < .001). Preincubation and stimulation of HMEC-1 cells in the presence of selective caspase inhibitors revealed that blocker for caspase-2 (z-VDVAD-FMK) inhibited thrombin-induced EMP release (Figure 5A, lane 12 vs lane 9; P < .01). It should be noted that inhibition of caspase-6 with its selective inhibitor z-VEID-FMK slightly inhibited thrombin-induced EMP release (Figure 5A, lane 14 vs lane 9; P < .01). No inhibition in thrombin-induced EMP release was found when HMEC-1 cells were stimulated with thrombin in the presence of the selective inhibitor of caspase-3, z-DVED-FMK (Figure 5A, lane 13 vs lane 9). It should be noted that the Pan-caspase and caspase-3 inhibitors slightly decreased the basal EMP release (Figure 5A, lane 2 and lane 5 vs lane 1; not significant). Caspase-2 activity in HMEC-1 lysates was then studied to ascertain a direct effect of thrombin on caspase-2. Thrombin induced a time-dependent increase in caspase-2 activity (Figure 5B, upper panel; P < .01) that peaked 3 hours after the onset of stimulation. Caspase-2 activity was abolished in presence of Z-VDVAD-FMK, the selective caspase-2 inhibitor (Figure 5B, lower panel; P < .001); in contrast, the Rho-kinase inhibitor Y27632 displayed no effect on the thrombin-induced caspase-2 activity.
Involvement of Caspase-2 in EMP release. (A) Inhibition of thrombin-induced-EMP release by caspase inhibitors. Serum-starved HMEC-1 cells were preincubated for 1 hour with 2 μM Pan-caspase inhibitor (Z-VAD-FMK) or each of the specific caspase inhibitor. Caspase-1 inhibitor: Z-YVAD-FMK; caspase-2 inhibitor: Z-VDVAD-FMK; caspase-3 inhibitor: Z-DVED-FMK; caspase-6 inhibitor: Z-VEID-FMK; caspase-8 inhibitor: Z-IETD-FMK; caspase-9 inhibitor: Z-LEHD-FMK. HMEC-1 were kept untreated or stimulated with 1 IU/mL thrombin in presence of the same concentrations of inhibitors for 18 hours. EMP release was determined as described in “Materials and methods.” Bar graphs represent mean values ± SEM for 3 independent experiments. *P < .05, **P < .01, ***P < .001; NS indicates not significant. (B) Activation of caspase-2 by thrombin. Caspase-2 activity was determined in triplicate wells by a fluorescent assay. Results are expressed in relative fluorescent units. The curves represent the mean values ± SEM from 3 independent experiments. Upper panel: time-dependent increase in caspase-2 activity. HMEC-1 cells were kept unstimulated (•) or stimulated with thrombin (♦) for different times and lysed. **P < .01 versus unstimulated cells at the same time. Lower panel: HMEC-1 cells were preincubated with 2 μM Z-VDVAD-FMK or 1 μM Y27632 and stimulated for 3 hours with 1 IU/mL thrombin in presence of the inhibitors. ***P < .001 versus thrombin-stimulated HMEC cells. (C) Absence of apoptosis in HMEC-1 cells stimulated by thrombin. Nucleosomes enrichment determined by cell death ELISA. Serum-starved HMEC-1 cells were kept untreated (control) or were stimulated for 18 hours with thrombin (1 IU/mL) or 25 ng/mL TNF-α and 2.5 μg/mL cyloheximide (TNF/CHX). ***P < .001; NS indicates not significant.
Involvement of Caspase-2 in EMP release. (A) Inhibition of thrombin-induced-EMP release by caspase inhibitors. Serum-starved HMEC-1 cells were preincubated for 1 hour with 2 μM Pan-caspase inhibitor (Z-VAD-FMK) or each of the specific caspase inhibitor. Caspase-1 inhibitor: Z-YVAD-FMK; caspase-2 inhibitor: Z-VDVAD-FMK; caspase-3 inhibitor: Z-DVED-FMK; caspase-6 inhibitor: Z-VEID-FMK; caspase-8 inhibitor: Z-IETD-FMK; caspase-9 inhibitor: Z-LEHD-FMK. HMEC-1 were kept untreated or stimulated with 1 IU/mL thrombin in presence of the same concentrations of inhibitors for 18 hours. EMP release was determined as described in “Materials and methods.” Bar graphs represent mean values ± SEM for 3 independent experiments. *P < .05, **P < .01, ***P < .001; NS indicates not significant. (B) Activation of caspase-2 by thrombin. Caspase-2 activity was determined in triplicate wells by a fluorescent assay. Results are expressed in relative fluorescent units. The curves represent the mean values ± SEM from 3 independent experiments. Upper panel: time-dependent increase in caspase-2 activity. HMEC-1 cells were kept unstimulated (•) or stimulated with thrombin (♦) for different times and lysed. **P < .01 versus unstimulated cells at the same time. Lower panel: HMEC-1 cells were preincubated with 2 μM Z-VDVAD-FMK or 1 μM Y27632 and stimulated for 3 hours with 1 IU/mL thrombin in presence of the inhibitors. ***P < .001 versus thrombin-stimulated HMEC cells. (C) Absence of apoptosis in HMEC-1 cells stimulated by thrombin. Nucleosomes enrichment determined by cell death ELISA. Serum-starved HMEC-1 cells were kept untreated (control) or were stimulated for 18 hours with thrombin (1 IU/mL) or 25 ng/mL TNF-α and 2.5 μg/mL cyloheximide (TNF/CHX). ***P < .001; NS indicates not significant.
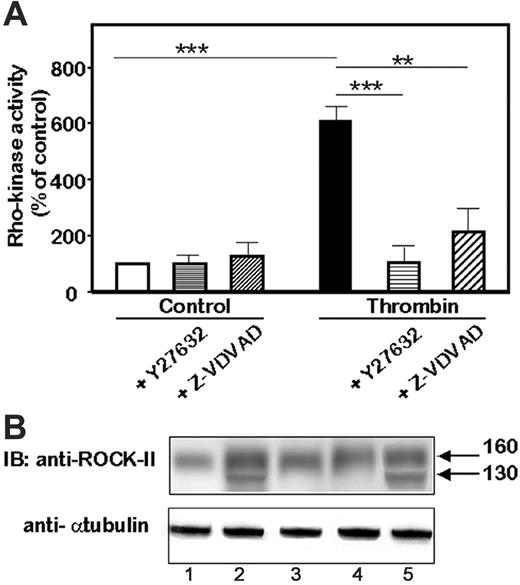
Involvement of caspase-2 in the activation of ROCK-II. (A) Rho-kinase activity in response to thrombin. Rho-kinase activity was determined by a colorimetric assay of the phosphorylation of MBS substrate. HMEC-1 cells were kept untreated or were stimulated for 4 hours with 1 IU/mL thrombin. **P < .01; ***P < .001. (B) Representative immunoblot of the effects of caspase inhibitors on the release of the 130 kDa cleaved form of ROCK-II. Immunoblots were performed after a 4-hour stimulation with thrombin. Cell lysates from unstimulated or thrombin-stimulated HMEC-1 cells were resolved on 4% to 12% PAGE and the blots were probed with an anti–ROCK-II monoclonal antibody or antitubulin as a loading control. Lane 1 is unstimulated HMEC-1; lane 2, thrombin-stimulated HMEC-1; lane 3, ZVAD-FMK; lane 4, Z-VDVAD-FMK; and lane 5, Z-DVED-FMK–treated HMEC-1.
Involvement of caspase-2 in the activation of ROCK-II. (A) Rho-kinase activity in response to thrombin. Rho-kinase activity was determined by a colorimetric assay of the phosphorylation of MBS substrate. HMEC-1 cells were kept untreated or were stimulated for 4 hours with 1 IU/mL thrombin. **P < .01; ***P < .001. (B) Representative immunoblot of the effects of caspase inhibitors on the release of the 130 kDa cleaved form of ROCK-II. Immunoblots were performed after a 4-hour stimulation with thrombin. Cell lysates from unstimulated or thrombin-stimulated HMEC-1 cells were resolved on 4% to 12% PAGE and the blots were probed with an anti–ROCK-II monoclonal antibody or antitubulin as a loading control. Lane 1 is unstimulated HMEC-1; lane 2, thrombin-stimulated HMEC-1; lane 3, ZVAD-FMK; lane 4, Z-VDVAD-FMK; and lane 5, Z-DVED-FMK–treated HMEC-1.
Caspases are well known to act as effectors of apoptotic cell death.33 The capacity of thrombin in inducing apoptosis in HMEC-1 cells was determined by quantitative determination of cytoplasmic histone-associated DNA fragments by Cell Death ELISA kit. Quantification of cytoplasmic nucleosomes indicated similar low levels in unstimulated or thrombin-stimulated HMEC-1 cells (Figure 5C; not significant). As a control, in HMEC-1 cells treated with 25 ng/mL TNF-α and 2.5 μg/mL cycloheximide, high levels of oligonucleosomes were detected (P < .001 vs control).
We hypothesized that caspase-2 played a role in ROCK-II cleavage. To gain insight into the role of caspase-2 in ROCK-II activation, we determined whether inhibition of caspase-2 induced changes in ROCK activation. Thrombin stimulation of HMEC-1 cells induced a net increase in the phosphorylation of the Rho-kinase substrate (Figure 6A; P < .001 vs control) that was totally prevented by pretreatment and stimulation of HMEC-1 cells with Y27632 1 μM (P < .001 vs thrombin-stimulated HMEC-1 cells). Z-VDVAD-FMK, the selective inhibitor, greatly inhibited the Rho-kinase activity in HMEC-1 cells stimulated by thrombin (P < .01 vs thrombin-stimulated HMEC-1 cells) and displayed no effect on control cells. We next determined whether caspase-2 was involved in the cleavage of ROCK-II. Immunoblots were performed on lysates from HMEC-1 cells pretreated and stimulated with thrombin in the presence of the caspase inhibitors (Figure 6B). The cleavage of ROCK-II leading to the approximately 130-kDa form was abolished in cells treated with z-VAD-FMK, the pancaspase inhibitor (Figure 6B, lane 3 vs lane 2) or with z-VDVAD-FMK, the caspase-2 blocker (Figure 6B, lane 4 vs lane 2). In contrast, the inhibition of caspase-3 by its inhibitor z-DVED-FMK did not prevent the thrombin-induced cleavage of ROCK-II (Figure 6B, lane 5 vs lane 2). Taken together, these data indicated that thrombin recruit caspase-2 despite an absence of cell death by apoptosis and that caspase-2 acted upstream of ROCK-II.
Discussion
Thrombin is a multifunctional enzyme that generates both beneficial physiologic responses and pathogenic events in endothelial cells.34 It exerts pleiotropic effects on endothelial cells, including EMP release.6 Nevertheless, the mechanisms leading to this release are not known. Our data provide evidence that thrombin stimulation of the microvascular endothelial cell line HMEC-1 induces a dose- and time-dependent release of EMPs by a signaling pathway depending on the RhoA/ROCK axis. Using gene expression profiling and gene silencing, this study identifies for the first time ROCK-II, but not ROCK-I, as a critical mediator of EMP generation by thrombin, presenting evidence that the 2 kinases are differently regulated. Interestingly, the demonstration of caspase-2 involvement in ROCK-II activation, despite an absence of cell death, unravels a novel knowledge of the mechanisms leading to EMP generation during cell activation.
The generation of EMPs by microvascular endothelial cells represents a novel strong signal of thrombin toward its endothelial target. The pathway leading to EMP release depends on the activation of PAR-1 because (1) the peptide agonist SFLLRN generated EMPs at a level equivalent to that of thrombin, and (2) nonproteolytic thrombin PPACK did not promote EMP release, indicating an absence of activation of PAR-2, another receptor sensitive to the proteolysis by trypsin but not by thrombin.35
Microparticle formation, a common mechanism of membrane shedding by activated cells, requires cytoskeleton rearrangement.6,36 The fact that thrombin generates EMPs by activating the axis RhoA/ROCK is consistent with a role of Rho-GTPases in the cytoskeletal changes leading to endothelial blebbing.37 The inhibition of thrombin-induced EMP release by the selective inhibitors Y-27632 or HA-107715 clearly establishes an involvement of the Rho-kinases in this pathway. ROCK-I and ROCK-II are 2 isoforms38,39 that have been assumed to perform the same functions.9 However, recent works discriminate between ROCK-I and ROCK-II in terms of distribution, cytoskeleton organization, and functions.40 Moreover, in ROCK-II–/––deficient mice, ROCK-I does not compensate for the loss of ROCK-II.41 Our study clearly identifies ROCK-II but not ROCK-I as a target of thrombin signaling. At first, microarray analysis indicates that, among the genes belonging to the functional family of cytoskeleton organization, ROCK-II is the most differentially expressed gene, whereas ROCK-I expression is not modulated. Second, ROCK-II protein increases with time in HMEC lysates whereas ROCK-I expression does not change. Third, ROCK-II but not ROCK-I is cleaved in response to thrombin stimulation. Our data clearly establish distinct actions between the 2 isoforms in endothelial cells, and confer to ROCK-II a major role in the control of the thrombin-induced EMP release. Indeed, silencing ROCK-II mRNA totally prevents the thrombin-induced EMP release and displays no significant effect on the basal release of EMPs. The cleavage of the C-terminal region of ROCK-I and ROCK-II is required for the full activation of their kinase activity. During apoptosis, bleb formation is controlled by ROCK-I cleavage by caspase-311,12 whereas ROCK-II remains uncleaved.42 During cytotoxic lymphocyte granule-induced cell death, ROCK-II is cleaved by granzyme B, by a caspase-independent pathway.13 In this work, we demonstrate that in response to thrombin, ROCK-II cleavage occurs in the absence of cell death in HMEC-1 cells. Nevertheless, it is yet mediated by the initiator caspase-2. Our data establish that caspase-2 is a target of thrombin and acts upstream of ROCK-II activation. In response to thrombin stimulation, caspase-2 activity is increased. This activity is required to activate ROCK-II. Indeed, the inhibition of caspase-2 activity by its selective inhibitor Z-VDVAD-FMK inhibits the thrombin-induced ROCK-II activity and prevents ROCK-II cleavage. Inhibition of caspase-2 significantly inhibits EMP release, indicating that the activation of ROCK-II by cleavage is a prerequisite step for the generation of EMPs in response to thrombin. All these data are in favor of a direct relationship among caspase-2 recruitment, ROCK-II activation, and EMP release. A link between thrombin and caspase activation has already been demonstrated in platelets with the association with the cytoskeleton of caspase-3 and -9 activation.43 Moreover, thrombin inhibits the cell growth of the human tumor cell line DU145 prostate by up-regulating the expression of caspases 1, 2, and 3.44 How caspase-2 is connected to ROCK cleavage and EMP generation is unclear at this stage. Recent data have shown that in response to cell stress, caspase-2 is recruited early in the apoptotic pathway, prior to mitochondrial permeabilization,45 and that its implication in cell death can be rescued by Bcl-2 protein.46 Our data on gene expression profiling clearly show an overexpression of the antiapoptotic genes BAG1 and BCL2L2, which could antagonize the apoptotic pathway triggered by caspase-2. Two other hypotheses could also explain the proteolytic activity of caspase-2. First, caspase-2 is a unique caspase that exists under 2 forms displaying antagonistic effects. The long form, Caspase2-L, induces cell death, whereas the short form, Caspase2-S, suppresses it.47,48 Currently, no information is available concerning the presence of the 2 forms in HMEC-1 cells. Second, caspase-2 has recently been involved in lipid synthesis or accumulation49 and in inflammation,50 2 effects independent of its involvement in the apoptotic pathway. Thus, the proteolytic activity of caspase-2 in response to thrombin represents a novel function of this enzyme, exceeding its well-known role in cell death.
Two cellular processes lead to the release of EMPs: apoptosis or cell activation.51 The mechanisms governing their formation are probably different, accounting for differences in terms of size and protein composition.52 Taken together, we add new insights into the mechanisms linking EMP release and cell activation. The involvement of ROCK-II and caspase-2 would allow differentiating microparticles generated by cell activation or apoptosis.
Importance of microparticles in the communication between cells is an emerging concept that confers to these vesicles a novel role in the control of vascular function. Their composition reflects the phenotypic characteristics of the parent cells from which they originated.20 More than simple cell fragments released in the blood stream, they behave as biovectors, allowing a transport at distance of parent cell molecules to other cells.53 The fact that thrombin promotes EMP generation may be of importance for the vascular biology. First, thrombin-induced EMPs could transfer endothelial information in a paracrine way to distant cells. Second, they could directly act on endothelial cells in an autocrine way17 to accelerate thrombin generation, so initiating an amplification loop54 that locally increases the thrombin action. Even if the procoagulant activity is the most frequently described characteristic of EMPs,18 not all their properties should be regarded as noxious.55,56 As thrombin promotes proliferative/migratory endothelial phenotype, EMPs may also prevent damage and favor vascular repair by mediating cell growth.
In conclusion, the present data describe a novel signaling cascade involved in EMP generation in cell activation. The absence of redundancy between the 2 Rho-kinases and the recruitment of caspase-2 independently of apoptosis emphasize the complexity of pathways that lead to the formation of microparticles. A better understanding of the mechanisms underlying microparticle formation from cell activation or apoptosis would contribute to delineate emergent therapeutic approaches.
Prepublished online as Blood First Edition Paper, May 23, 2006; DOI 10.1182/blood-2006-04-014175.
Supported by INSERM grants, Assistance Publique des Hôpitaux de Marseille, PHRC no. 02/07, the Université de la Méditerranée, and Diagnostica Stago. C.S. and S.S. received support from Assistance Publique des Hôpitaux de Marseille and Diagnostica Stago/Convention Industrielle de Formation pour la Recherche (CIFRE), respectively. The authors have no conflicting financial interests.
C.S. and S.S. contributed equally to this work.
C.S. performed research; S.S. performed research; D.P. analyzed bioinformatic data; B.L. contributed to cDNA microarray design; J.S. analyzed data; C.N. provided cDNA microarray and analyzed bioinformatic data; F.D.-G. analyzed data; and F.A. designed research and wrote the paper.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Dr P. Chavrier (Institut Pasteur, Paris, France) for his gift of RhoA plasmids. The authors thank Diagnostica Stago for its help in performing cDNA arrays.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal