Abstract
Hematide is an investigational pegylated synthetic peptide that stimulates erythropoiesis in animal models and is being developed for the treatment of anemia associated with chronic renal failure and cancer. This study evaluated the safety and pharmacodynamics of single, intravenous doses (0.025, 0.05, and 0.1 mg/kg) of Hematide in 28 healthy male volunteers. All doses of Hematide were well tolerated, with safety profiles similar to those of placebo. Hematide showed a dose-dependent increase in reticulocytes. The 0.1-mg/kg dose was associated with a statistically significant increase in hemoglobin (Hgb) from baseline compared to the placebo group (13.6 ± 3.9 g/L [1.36 ± 0.39 g/dL] versus 3.9 ± 3.8 g/L [0.39 ± 0.38 g/dL]; P < .001) that was sustained for longer than 1 month. These results support phase 2 studies in patients with anemia associated with chronic kidney disease or cancer and suggest that Hematide administered as infrequently as once a month may result in a sustained elevation of Hgb levels. (Please note that Hematide is a proposed trade name; the compound does not yet have a nonproprietary name.)
Introduction
There are several common clinical situations in which anemia is the result of hypoproliferation of red blood cell (RBC) precursors secondary to erythropoietin (EPO) deficiency. These include anemias associated with chronic kidney disease (CKD), chronic inflammation, and cancer, with or without myelosuppressive therapy. In these situations, recombinant human EPO (rHuEPO) has been used successfully to increase hemoglobin (Hgb) levels, reduce fatigue, and improve daily function.
Hematide is a synthetic, dimeric peptidic erythropoiesis-stimulating agent (ESA) covalently linked to polyethylene glycol (PEG) and is being developed for the treatment of anemia associated with chronic renal failure and cancer. Because its primary amino acid sequence is unrelated to that of rHuEPO, Hematide is unlikely to induce a cross-reactive immune response against endogenous EPO.1 This potentially reduces the risk of pure red cell aplasia (PRCA), a rare complication caused by an immune response to rHuEPO. PRCA reached a peak incidence outside of the United States in 2001 to 2002, largely related to a change in formulation of the product.2,3
Hematide binds to and activates the human EPO receptor, stimulating the proliferation and differentiation of human red cell precursors in vitro in a manner similar to ESAs.1 A predictable, dose-related effect on reticulocyte and Hgb levels has been observed in rats and monkeys.1 The design of this first study of Hematide in humans was based on these nonclinical data and the published reports of ESAs in approximately 400 healthy volunteers.4-13 This study was designed to evaluate the safety and pharmacokinetic and pharmacodynamic profiles of single intravenous dose levels of the drug and to determine the minimum pharmacologic active dose (PAD) in healthy subjects. Results of the pharmacokinetic analysis will be published separately.
Subjects, materials, and methods
Eligibility
Eligible subjects were men, 18 to 40 years of age, with a body mass index (BMI) of 18 to 30 kg/m2, without any clinically significant medical condition. At study entry, subjects were to have a Hgb value of 160 g/L (16 g/dL) or less, and normal values for ferritin, white blood cell count, and platelet count. All subjects were informed of the investigational nature of this study and signed informed consent. The study was approved by the local institutional review board and was conducted in accordance with guidelines for good clinical practice, the Declaration of Helsinki, and all applicable national and local regulations and requirements.
Study design
This was a phase 1, randomized, double-blind, placebo-controlled trial, conducted at a single clinical center in 28 healthy men. Hematide was evaluated at doses of 0.025, 0.05, and 0.1 mg/kg and compared to placebo. Treatment cohorts consisted of 7 subjects randomly assigned to receive either a single dose of Hematide or matching placebo in a 5:2 ratio. All subjects and the study team were blinded regarding subject assignment. Each dose of Hematide or placebo was administered via a 5-minute intravenous infusion on the day of dosing. Cohorts were evaluated sequentially, and dose escalation in each subsequent cohort was allowed based on prespecified safety criteria.
All subjects received 100 mg oral elemental iron daily supplied as ferrous fumarate beginning at screening and continuing through 28 days after receiving Hematide. Hgb levels and reticulocyte counts were measured at baseline, 3 times a week for 28 days, and twice a week thereafter until Hgb levels returned to within 5 g/L (0.5 g/dL) from baseline. In addition, complete blood count (CBC) with differential and platelet count, serum ferritin, transferrin saturation, soluble transferrin receptor protein, content of reticulocyte Hgb, and endogenous EPO were monitored weekly. All samples were analyzed in one local laboratory using the same automated equipment.
Safety was evaluated through adverse events, clinical laboratory assessments, vital signs, electrocardiographic (ECG) recordings, and physical examinations. The severity of each adverse event was blindly graded by the investigator using the World Health Organization's common toxicity criteria.
Anti-Hematide antibody determination
Anti-Hematide antibody determination was performed using an enzymelinked immunosorbent assay (ELISA) with wells directly coated with Hematide followed by application of 1:10 diluted sera with subsequent detection with goat anti–human immunoglobulins alkaline phosphatase conjugate (no. AHI0705; Biosource International, Camarillo, CA). The validated sensitivity of the assay for Hematide-specific IgG was 300 ng/mL, as estimated using a positive control mouse monoclonal antibody to Hematide.
Statistical analyses
Baseline Hgb was defined as the mean of the screening, day–1, and day 1 Hgb values. For all other parameters, the baseline value was the value reported on the day of injection (prior to dosing). Using the trapezoidal rule, areas under the response-time curve (AUCs) over 14 days or 28 days after the dosing were approximated for each subject for reticulocyte counts and for Hgb level. Comparisons between groups were made using one-way analysis of variance (ANOVA).
Results
Twenty-eight healthy men, ranging in age from 20 to 36 years, were enrolled in the study at a single site in the United Kingdom. The subjects were predominantly (79%) white, with BMIs ranging from 20 to 29 kg/m2. Four cohorts were enrolled in a consecutive manner and received Hematide doses of 0.025, 0.05, and 0.1 mg/kg (single dose per cohort). Dose-escalation stopping criteria for Hgb levels were achieved with the third cohort (0.1 mg/kg Hematide). This dose was repeated in the fourth cohort to confirm the results observed in the third cohort, after which the study was terminated. Altogether, 20 subjects were randomized to receive Hematide (5 in each of the 0.025- and 0.05-mg/kg treatment groups and 10 in the 0.1-mg/kg treatment group) and 8 subjects were randomized to the placebo group. Demographic and baseline characteristics were generally balanced across treatment groups (Table 1).
Demographics and baseline characteristics
. | Treatment group . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | Placebo . | 0.025 mg/kg . | 0.05 mg/kg . | 0.1 mg/kg . | |||
| No. subjects | 8 | 5 | 5 | 10 | |||
| Mean age, y, ± SD | 27.0 ± 5.3 | 25.2 ± 3.3 | 25.8 ± 5.3 | 28.2 ± 4.1 | |||
| Mean BMI, kg/m2, ± SD | 23.9 ± 1.7 | 23.2 ± 2.7 | 25.6 ± 1.5 | 25.7 ± 1.6 | |||
| Mean weight, kg, ± SD | 74.8 ± 10.3 | 72.4 ± 10.2 | 77.9 ± 5.0 | 82.4 ± 6.6 | |||
| Race, no. (%) | |||||||
| White | 4 (50) | 5 (100) | 4 (80) | 9 (90) | |||
| African | 2 (25) | 0 | 0 | 1 (10) | |||
| Asian | 2 (25) | 0 | 1 (20) | 0 | |||
| Baseline values, mean ± SD | |||||||
| Hemoglobin level, g/L | 149.0 ± 9.0 | 153.0 ± 8.0 | 151.0 ± 6.0 | 148.0 ± 7.0 | |||
| Ferritin concentration, μg/L | 131.1 ± 20.5 | 132.3 ± 22.4 | 186.4 ± 112.5 | 171.3 ± 73.9 | |||
| EPO level, IU/L | 12.1 ± 3.5 | 10.0 ± 2.4 | 13.0 ± 5.2 | 9.3 ± 1.8 | |||
. | Treatment group . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | Placebo . | 0.025 mg/kg . | 0.05 mg/kg . | 0.1 mg/kg . | |||
| No. subjects | 8 | 5 | 5 | 10 | |||
| Mean age, y, ± SD | 27.0 ± 5.3 | 25.2 ± 3.3 | 25.8 ± 5.3 | 28.2 ± 4.1 | |||
| Mean BMI, kg/m2, ± SD | 23.9 ± 1.7 | 23.2 ± 2.7 | 25.6 ± 1.5 | 25.7 ± 1.6 | |||
| Mean weight, kg, ± SD | 74.8 ± 10.3 | 72.4 ± 10.2 | 77.9 ± 5.0 | 82.4 ± 6.6 | |||
| Race, no. (%) | |||||||
| White | 4 (50) | 5 (100) | 4 (80) | 9 (90) | |||
| African | 2 (25) | 0 | 0 | 1 (10) | |||
| Asian | 2 (25) | 0 | 1 (20) | 0 | |||
| Baseline values, mean ± SD | |||||||
| Hemoglobin level, g/L | 149.0 ± 9.0 | 153.0 ± 8.0 | 151.0 ± 6.0 | 148.0 ± 7.0 | |||
| Ferritin concentration, μg/L | 131.1 ± 20.5 | 132.3 ± 22.4 | 186.4 ± 112.5 | 171.3 ± 73.9 | |||
| EPO level, IU/L | 12.1 ± 3.5 | 10.0 ± 2.4 | 13.0 ± 5.2 | 9.3 ± 1.8 | |||
All 28 subjects completed the first 28-day portion of the study; 3 subjects (one in the placebo group and 2 in the 0.1-mg/kg group) completed all assessments through 28 days after the dosing but did not return consistently for further follow-up assessments. All 28 subjects were included in the safety analysis; however, only 27 of the 28 subjects were included in the pharmacodynamic analyses. During study drug administration, one subject (in the 0.025-mg/kg group) experienced an adverse event that led to discontinuation of the infusion. As a result, the subject received approximately 40% of the intended dose of Hematide and results from this subject were excluded from the pharmacodynamic analyses.
Pharmacodynamics
Reticulocytes reached a maximum approximately 7 days after the dose and returned to within baseline values approximately 2 weeks after dosing in all dose cohorts. There was a statistically significant, dose-dependent increase in reticulocytes across all dose levels (Figure 1; P < .001; Table 2).
Cmax and AUC0-14 reticulocyte counts by dose group
. | Dose group . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | Placebo . | 0.025 mg/kg . | 0.05 mg/kg . | 0.1 mg/kg . | F test* . | P . | |||
| No. subjects | 8 | 4 | 5 | 10 | NA | NA | |||
| Mean Cmax, 109/L, ± SD | 61 ± 16 | 148 ± 39 | 186 ± 44 | 227 ± 41 | 34.2 | < .001 | |||
| Mean AUC0-14, 109/L × d, ± SD | 661 ± 205 | 1390 ± 439 | 1502 ± 273 | 1932 ± 416 | 20.3 | < .001 | |||
. | Dose group . | . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | Placebo . | 0.025 mg/kg . | 0.05 mg/kg . | 0.1 mg/kg . | F test* . | P . | |||
| No. subjects | 8 | 4 | 5 | 10 | NA | NA | |||
| Mean Cmax, 109/L, ± SD | 61 ± 16 | 148 ± 39 | 186 ± 44 | 227 ± 41 | 34.2 | < .001 | |||
| Mean AUC0-14, 109/L × d, ± SD | 661 ± 205 | 1390 ± 439 | 1502 ± 273 | 1932 ± 416 | 20.3 | < .001 | |||
NA indicates not applicable.
One-way ANOVA.
The Hgb response (Figure 2A) and maximum change from baseline (Figure 3) also showed significant differences across dose groups (P < .001). Although there was not a significant increase in Hgb concentration in the 2 lower dose groups, the 0.1-mg/kg dose group demonstrated a statistically significant increase in Hgb level from baseline, with an average maximum increase from baseline of 13.6 g/L (SD, 3.9; range, 7.0-19.0; [1.36 g/dL]) for the 10 Hematide recipients versus 3.9 g/L (SD, 3.8; [0.39 g/dL]) for the placebo recipients, P < .001 (Figure 3). Subjects in the 0.1-mg/kg dose group and concurrent placebo subgroup were followed through 42 days, with Hgb values in the Hematide-treated group remaining 5.0 g/L (0.5 g/dL) or higher over controls throughout this period (data not shown).
Mean dose-dependent reticulocyte response following Hematide dosing. ▵, placebo; □, 0.025 mg/kg; ▴, 0.05 mg/kg; and ▪, 0.1 mg/kg. P < .001 for Cmax and AUC0-14 among dose groups.
Mean dose-dependent reticulocyte response following Hematide dosing. ▵, placebo; □, 0.025 mg/kg; ▴, 0.05 mg/kg; and ▪, 0.1 mg/kg. P < .001 for Cmax and AUC0-14 among dose groups.
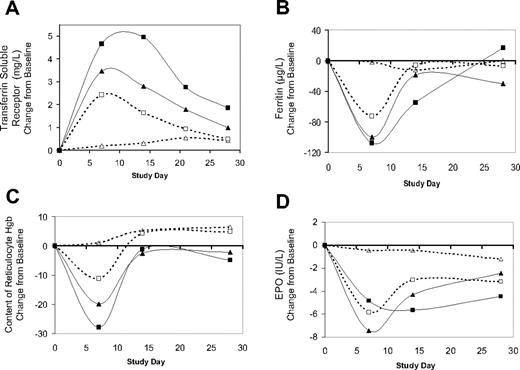
After Hematide dosing, changes were observed in other measures of the erythropoietic response. RBC count (Figure 2B), hematocrit, (data not shown), and soluble transferrin receptor protein (Figure 4A) all increased in a dose-dependent manner. Decreases in ferritin (Figure 4B) and reticulocyte Hgb content (Figure 4C) were detected in all 3 dose groups at day 7 and returned to baseline values thereafter.
Subjects who had an increase in Hgb were more likely to have serum EPO levels that fell below the lower limit of the normal range (which is 5-24.6 U/L); this occurred in 2 of the 4 subjects in the 0.025-mg/kg group, 3 of 5 subjects in the 0.05-mg/kg group, and all 10 subjects in the 0.1-mg/kg group. By 7 days after dosing, the mean change from baseline reached a nadir for the 0.025-mg/kg and 0.05-mg/kg dose groups, compared with 14 days after dosing in the 0.1-mg/kg group (Figure 4D). By 28 days after dosing, EPO levels were generally within normal limits in the 0.025- and 0.05-mg/kg dose groups, with median EPO levels of 6.9 and 7.5 U/L, respectively, but remained low in the 0.1-mg/kg dose group, which had a median EPO level of 4.9 U/L, correlating with the sustained Hgb increase in this group.
Mean change from baseline Hgb and RBC counts. Panel A represents baseline Hgb (P < .001 among dose groups); panel B, the RBC counts through 28 days following Hematide dosing. ▵, placebo; □, 0.025 mg/kg; ▴, 0.05 mg/kg; ▪ 0.1 mg/kg.
Mean change from baseline Hgb and RBC counts. Panel A represents baseline Hgb (P < .001 among dose groups); panel B, the RBC counts through 28 days following Hematide dosing. ▵, placebo; □, 0.025 mg/kg; ▴, 0.05 mg/kg; ▪ 0.1 mg/kg.
There were no significant changes in mean corpuscular volume, mean corpuscular Hgb concentration, or mean corpuscular Hgb levels of red blood cells (data not shown).
Safety
Hematide was generally well tolerated in this population. The type, frequency, and severity of adverse events observed in Hematide-treated subjects appeared similar to those of placebo-treated subjects (Table 3).
Type and frequency of adverse events
. | Treatment group* . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Adverse event . | Placebo . | 0.025 mg/kg . | 0.05 mg/kg . | 0.1 mg/kg . | |||
| No. subjects | 8 | 5 | 5 | 10 | |||
| Cardiac disorder: palpitations | 0 | 1 (20.0) | 0 | 0 | |||
| Gastrointestinal disorders | |||||||
| Abdominal distention | 0 | 1 (20.0) | 0 | 0 | |||
| Abdominal pain | 0 | 1 (20.0) | 0 | 1 (10.0) | |||
| Constipation | 0 | 1 (20.0) | 0 | 0 | |||
| Diarrhea | 0 | 1 (20.0) | 0 | 0 | |||
| Nausea | 0 | 0 | 0 | 2 (20.0) | |||
| General disorders | |||||||
| Fatigue | 0 | 1 (20.0) | 0 | 0 | |||
| Thirst | 0 | 0 | 0 | 1 (10.0) | |||
| Immune system disorder: drug hypersensitivity | 0 | 1 (20.0) | 0 | 0 | |||
| Infection: nasopharyngitis | 3 (37.5) | 0 | 0 | 2 (20.0) | |||
| Injury | |||||||
| Head injury | 0 | 0 | 0 | 1 (10.0) | |||
| Joint sprain | 0 | 0 | 0 | 1 (10.0) | |||
| Limb injury | 1 (12.5) | 0 | 0 | 0 | |||
| Musculoskeletal disorder: back pain | 0 | 0 | 0 | 1 (10.0) | |||
| Nervous system disorders | |||||||
| Dizziness | 0 | 0 | 0 | 1 (10.0) | |||
| Headache | 1 (12.5) | 1 (20.0) | 0 | 3 (30.0) | |||
| Syncope vasovagal | 1 (12.5) | 0 | 0 | 0 | |||
| Reproductive system disorder: | |||||||
| testicular disorder | 0 | 0 | 0 | 1 (10.0) | |||
. | Treatment group* . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Adverse event . | Placebo . | 0.025 mg/kg . | 0.05 mg/kg . | 0.1 mg/kg . | |||
| No. subjects | 8 | 5 | 5 | 10 | |||
| Cardiac disorder: palpitations | 0 | 1 (20.0) | 0 | 0 | |||
| Gastrointestinal disorders | |||||||
| Abdominal distention | 0 | 1 (20.0) | 0 | 0 | |||
| Abdominal pain | 0 | 1 (20.0) | 0 | 1 (10.0) | |||
| Constipation | 0 | 1 (20.0) | 0 | 0 | |||
| Diarrhea | 0 | 1 (20.0) | 0 | 0 | |||
| Nausea | 0 | 0 | 0 | 2 (20.0) | |||
| General disorders | |||||||
| Fatigue | 0 | 1 (20.0) | 0 | 0 | |||
| Thirst | 0 | 0 | 0 | 1 (10.0) | |||
| Immune system disorder: drug hypersensitivity | 0 | 1 (20.0) | 0 | 0 | |||
| Infection: nasopharyngitis | 3 (37.5) | 0 | 0 | 2 (20.0) | |||
| Injury | |||||||
| Head injury | 0 | 0 | 0 | 1 (10.0) | |||
| Joint sprain | 0 | 0 | 0 | 1 (10.0) | |||
| Limb injury | 1 (12.5) | 0 | 0 | 0 | |||
| Musculoskeletal disorder: back pain | 0 | 0 | 0 | 1 (10.0) | |||
| Nervous system disorders | |||||||
| Dizziness | 0 | 0 | 0 | 1 (10.0) | |||
| Headache | 1 (12.5) | 1 (20.0) | 0 | 3 (30.0) | |||
| Syncope vasovagal | 1 (12.5) | 0 | 0 | 0 | |||
| Reproductive system disorder: | |||||||
| testicular disorder | 0 | 0 | 0 | 1 (10.0) | |||
Except for the “No. patients” row, all data are presented as number of subjects experiencing adverse event (%).
Fifteen subjects (4 of 8 placebo recipients and 11 of 20 Hematide recipients) experienced a total of 28 adverse events (6 in the placebo group and 22 in the Hematide dose groups). Twenty-seven adverse events were graded as mild (grade 1). The only grade 2 adverse event was a moderate head injury, not related to study drug, which occurred 22 days after the dosing. Of the 22 adverse events reported by the Hematide recipients, 7 events were reported to be possibly associated with Hematide. They occurred in 3 subjects (2 in the 0.025-mg/kg and 1 in the 0.1-mg/kg dose groups) and were all of mild severity. These events were palpitations, nausea, fatigue, drug reaction, dizziness, and headache. There was no difference in frequency, severity, or pattern of adverse events among the 4 treatment groups (3 Hematide groups and the placebo group). There were no serious adverse events, and no subject withdrew from the study due to an adverse event. However, study drug infusion was discontinued in one subject (in the 0.025-mg/kg dose group) due to an apparent mild drug reaction characterized by flushing and a scratchy throat, which occurred approximately 2 minutes after the start of infusion. The symptoms resolved rapidly and spontaneously after discontinuation of the infusion. One placebo recipient and one Hematide recipient were given concomitant medications for mild headache and mild abdominal cramps, respectively. No clinically significant changes occurred in vital signs, ECG recordings, or laboratory values.
Mean maximum change from baseline Hgb at any time following Hematide dosing. Error bars reflect SDs. P < .001 among dose groups.
Mean maximum change from baseline Hgb at any time following Hematide dosing. Error bars reflect SDs. P < .001 among dose groups.
Hematide produced dose-dependent changes in pharmacodynamic parameters. Increase in transferrin soluble receptor (A) and decreases in ferritin (B), content of reticulocyte Hgb (C), and EPO (D). Each panel shows the change from baseline for each parameter over 28 days following Hematide dosing; baseline values were measured immediately prior to Hematide dosing. The symbols in each panel correspond to Hematide doses as follows: ▵, placebo; □, 0.025 mg/kg; ▴ 0.05 mg/kg; ▪, 0.1 mg/kg.
Hematide produced dose-dependent changes in pharmacodynamic parameters. Increase in transferrin soluble receptor (A) and decreases in ferritin (B), content of reticulocyte Hgb (C), and EPO (D). Each panel shows the change from baseline for each parameter over 28 days following Hematide dosing; baseline values were measured immediately prior to Hematide dosing. The symbols in each panel correspond to Hematide doses as follows: ▵, placebo; □, 0.025 mg/kg; ▴ 0.05 mg/kg; ▪, 0.1 mg/kg.
All subjects tested negative for antibodies to Hematide at day 28.
Discussion
In this group of subjects, Hematide was well tolerated with a safety profile that appeared comparable to placebo. There was no apparent increase in frequency or severity of any adverse event with higher doses of Hematide. This benign safety profile is consistent with that of other ESAs administered to healthy volunteers.5-13
Hematide showed dose-dependent increases in reticulocytes with greater and more sustained responses with increasing dose. The 0.1-mg/kg dose was determined to be the PAD in healthy volunteers because it was associated with a statistically and biologically significant increase in Hgb from baseline that was sustained for longer than 1 month. The magnitude and duration of the erythropoietic response in Hgb after a single injection in all subjects at that dose are remarkable compared to results with rHuEPO. Changes in Hgb after a single rHuEPO injection in healthy volunteers are transient or require multiple injections.5,6 The effect is presumably due to the short circulating half-life of rHuEPO and lack of a robust, sustained erythropoietic response following only one or 2 doses.4,5 Prolonged half-life and slow clearance have been described with Hematide in several animal species1 and have been confirmed in this study (data not shown). This likely contributes to the magnitude of the effect of a single dose of Hematide compared to a single dose of rHuEPO.
It appears that a sufficient increase in the magnitude and duration of the reticulocyte response (as measured by the AUC for reticulocytes over time and shown in Table 2 and Figure 1) is required to observe an increase in Hgb following the administration of Hematide or rHuEPO.4,5 Our data suggest that Hematide is a potent ESA that is likely to require infrequent dosing to correct Hgb levels in patients with chronic anemia. Recent data14 indicate that increases in Hgb levels are sustained for at least 1 month (at levels comparable to those observed at 0.1 mg/kg in healthy subjects) in ESA-naïve anemic patients with CKD who received 0.05 mg/kg Hematide. These data suggest that anemia may be corrected in patients with CKD with Hematide injections administered as infrequently as once every 4 weeks.
The transient dose-related reduction in endogenous EPO levels observed in this trial in response to Hematide is the expected physiologic response to an increase in Hgb as a result of exogenous erythropoiesis stimulation.15 The exact timing of the nadir in EPO levels could not be precisely determined due to the weekly testing frequency. The decrease in EPO levels is most pronounced and sustained in the subjects who received the 0.1 mg/kg dose of Hematide due to the higher and more prolonged increase in Hgb levels. Transient changes in other pharmacodynamic parameters (increases in RBC count, hematocrit value, and soluble transferrin receptor protein, and decreases in ferritin, reticulocyte Hgb content) are also consistent with stimulation of erythropoiesis in healthy iron-replete volunteers.4,5,11 Rapid mobilization of iron is required for a robust Hgb response during erythropoietic stimulation with exogenous ESAs. Healthy volunteers receiving single or repeat injections of rHuEPO, even with oral or intravenous iron supplementation, have been shown to have transient decreases in the content of reticulocyte Hgb and serum ferritin levels.4,5,11,16-18 In our study, subjects were required to have normal ferritin levels at entry, received oral iron replacement, and experienced only limited stimulation of erythropoiesis following one injection. The transient decrease in the content of Hgb in reticulocytes (CHr) and ferritin levels, also reported in studies of rHuEPO, presumably reflects the acute mobilization of iron to the erythroid compartment. During this significant increase in erythropoiesis, it appears that iron cannot be released from tissue iron stores rapidly enough to maintain normal CHr, indicating that there is a transient state of functional iron deficiency even in healthy, iron-replete individuals receiving oral iron.
Based on the results of this study, single and repeat dose phase 2 studies of Hematide in patients with anemia associated with CKD and cancer have been initiated.
Prepublished online as Blood First Edition Paper, May 23, 2006; DOI 10.1182/blood-2006-04-015818.
Supported by Affymax, Inc.
Several of the authors (J.S.I., K.K.L., K.W.W., P.J.S., R.N., and A.M.D.) are employed by a company (Affymax) whose potential product was studied in the present work. Two of the authors (R.B.S. and D.M.O.) were consultants for Affymax.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Deborah M. Lidgate for professional assistance in preparing the manuscript and Min-Jia Chen for developing, validating, and testing serum samples with the ELISA used to detect Hematide-specific antibodies.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal