Human immunodeficiency virus (HIV)-specific CD4+ lymphocytes are preferentially infected in HIV-positive individuals. To study this preferential infection, we have derived several HIV-specific (HS) CD4+ clones. We show that in dendritic cells (DCs), HIV virion capture led to major histocompatibility complex class-II (MHC-II)-restricted viral antigen presentation and to activation of HS cells. In contrast, neither cell-free virions nor infected lymphocytes activated HS cells. In DCs, the dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN/CD209), which internalizes virions, promoted MHC-II presentation of HIV antigens. Activation of HS cells by HIV-exposed DCs triggered an efficient viral spread in lymphocytes. CD4+ clones with irrelevant antigenic specificities were not activated by HIV-exposed DCs and poorly supported viral replication under this setting. Our results unravel the mechanisms of MHC-II-restricted HIV antigen presentation by DCs and describe how HIV gains access to the very cells designed by the immune system to counteract this pathogen.
Introduction
Acquired immunodeficiency syndrome (AIDS) is the result of a constant struggle between human immunodeficiency virus (HIV) and the immune system. The major virus-producing cells in the host are memory CD4+ T lymphocytes. HIV also infects, at lower levels, dendritic cells (DCs) and macrophages. Among memory CD4+ T cells, HIV preferentially targets HIV-specific (HS) lymphocytes: in vivo, HS cells harbor viral DNA sequences at a frequency about 4 times higher than lymphocytes of other antigenic specificities.1 HS cells thus contribute substantially to the infected T-cell pool. Besides being targeted by the virus, HS CD4+ cells are functionally compromised. These cells display defects in proliferative capacities and IL-2 production.2-4 HS proliferating responses can be rescued by adding anti-CD28 antibodies or IL-2, indicating that these cells are present but dysfunctional.5
How HIV gets access to HS CD4+ lymphocytes is not fully understood. It has been proposed that DCs, which efficiently capture HIV particles, could both deliver signals necessary for activating CD4+ T cells and transfer viral infection to responding cells. By using cytomegalovirus (CMV) as a model antigen, Loré et al6 recently demonstrated that in DC-T-cell cocultures, in the presence of exogenous CMV antigens, DCs preferentially transfer HIV to CMV-specific CD4 T cells. However, a direct analysis of HIV transfer from DCs to HS CD4+ cells has not yet been performed, in large part because of the difficulties in growing sufficient amounts of CD4 lymphocytes recognizing HIV epitopes.
DCs orchestrate innate and adaptive immune responses.7 HIV-1 subverts the migration properties of DCs to gain access to CD4+ T cells in lymph nodes.8 DCs express HIV-1 receptors (CD4, CCR5, and CXCR4), allowing productive infection of the cells.9-11 DCs are also equipped with various molecules involved in HIV-1 capture. Among them, the lectin DC-SIGN (CD209) binds gp120, the viral envelope, with high affinity.12 DC-SIGN is expressed by certain subsets of mucosal DCs,13 enhances infection of DCs, and facilitates viral transfer to T cells.11,14-16 Transmission of virions from DCs to CD4+ T cells follows a biphasic mode. Within hours following HIV-1 exposure, DCs directly transfer incoming virions, whereas at later time points, only newly synthesized viruses will be transmitted.11,17 Virus transfer within DC-T-cell conjugates is an efficacious process, involving the formation of a so-called infectious or viral synapse.18-20
Little is known about the cellular mechanisms leading to MHC-II-restricted presentation of HIV antigens by DCs, and the subsequent activation of HS lymphocytes. The cellular receptors involved, the pathways of viral capture, and antigen processing are not characterized. DCs efficiently cross-present HIV antigens, derived from incoming virions or from infected dying cells.21-27 This cross-presentation leads to the activation of anti-HIV CD8+ cytotoxic T lymphocytes (CTLs). We previously reported that DC-SIGN promotes HIV-1 virion capture and subsequent MHC-I-restricted antigen presentation.28 Various lectins are also involved in antigen uptake leading to MHC-II presentation.29-31 However, most, if not all, studies about lectins have been performed using model antigens (antibodies, mannosylated proteins). For instance, DC-SIGN has been shown to present an IgG-derived antigen to anti-IgG CD4+ T cells.32 Whether DC-SIGN also promotes MHC-II presentation of HIV antigens is not known.
We have studied here the pathways of MHC-II presentation of HIV antigens by DCs, the role of DC-SIGN in this process, and the parameters of cellular activation and viral infection of HS CD4 T cells. To address these points, we generated several HS CD4+ clones and, as controls, cells with irrelevant antigenic specificities. We demonstrate that DCs are key actors of HIV spread, activating HS cells and then delivering the cytopathic virus to the very cells designed by the immune system to counteract this pathogen.
Materials and methods
Cells and viruses
Clinical-grade DCs were prepared using a VacCell processor (Vac Cell, Paris, France) as described.33 Peripheral blood mononuclear cells (PBMCs) were cultured for 7 days in serum-free medium (InVitrogen, Frederick, MD) with 500 U/mL GM-CSF (Gentaur, Brussels, Belgium) and 50 ng/mL IL-13 (Peprotech, Tebu-bio, Rocky Hill, NJ), and DCs were isolated by elutriation. DCs used to derive the IV9 clone were generated using 1000 U/mL IL-4 (R&D, Minneapolis, MN) and 100 ng/mL GM-CSF (Gentaur) as described.34 Both isolation procedures yielded immature DCs (CD1a+, MHC-I+, MHC-II+, CD64-, CD83-, CD80-low, CD86-low cells). CD4+ T lymphocytes were isolated untouched from PBMCs using magnetic beads (Miltenyi Biotec, Auburn, CA) and cryopreserved. B cells were immortalized using Epstein-Barr virus (EBV) from infected B95-8 cells (a kind gift from Florence Buseyne) and cultured with 2 μg/mL cyclosporine A (Sigma, St Louis, MO). B-lymphoid lines expressing DC-SIGN WT or LL/AA mutant were derived by transduction with lentiviral vectors and sorting of DC-SIGN+ cells.35 HIV and HIV (vesicular stomatitis virus [VSV]) virions were produced as described.36 Env-deleted (HIVΔenv) and WT isogenic HIVBRU viruses were produced in activated CD4+ T cells using VSV-pseudotyped particules. AT-2-inactivated HIVMN (HIVMN-AT2) was kindly provided by J. D. Lifson (AIDS Vaccine Program, Science Applications International Corporation-Frederick, National Cancer Institute, Frederick, MD).37
Generation of HIV-specific CD4+ T cells by in vitro priming
Immature DCs (ImDCs, 2 × 106) were pulsed for 2 hours at 37°C with HIVMN-AT2 (1000 ng/mL or 20 nM p24) in the presence of cytokines (IL-4 or IL-13 and GM-CSF). Subsequently, maturation agents (IFNγ, 500 U/mL [Imukin; Boehringer Ingelheim, Mannheim, Germany] and ribomunyl, 1 μg/mL [Pierre Fabre Medicament]) were added for 4 hours. DCs were then washed and seeded in 96-well plates (2 × 104/well) with autologous CD4+ T cells (105/well) and irradiated autologous feeder cells (CD4-monocyte-PBMC fraction, 4 × 104/well) in RPMI 1640 medium supplemented with 5% human serum (Institut Jacques Boy, Paris, France), l-glutamine (2 mM), sodium pyruvate (10 mM), nonessential amino acids (1 ×), and Hepes (10 mM; Gibco, Carlsbad, CA). Recombinant human IL-2 (rhIL-2, 100 IU/mL, proleukin; Chiron, Emeryville, CA) was added 5 days later. Ten days after initial contact, cells were restimulated using autologous imDCs (1:20, DC/CD4+ T-cell ratio) pulsed with a cocktail of HIV-1 p24-Gag peptides and irradiated autologous feeder cells (5:1, feeder/CD4+ T-cell ratio). These peptides were previously selected for their immunogenicity in HLA-DRβ*01 transgenic mice (A.P., manuscript in preparation) or were already published as HLA-DRβ*04 binders.38 Peptide specificities of the cell lines were tested 15 days later, using [3H]-thymidine proliferation assays. Cell lines demonstrating peptide-specific responses were selected to generate clones by limiting dilution. Autologous PBMCs were activated with pokeweed mitogen for 48 hours at 37°C (10 μg/mL; Sigma) and subsequently used as stimulator cells. CD4+ cells were seeded in a 96-well plate with irradiated pokeweed blasts (4 × 104/mL well) pulsed with peptide (10 μg/mL) and feeder cells (1 × 105/well) in the presence of PHA (0.25 μg/mL; Abbott Murex, Abbott Park, IL) and rhIL-2. Clones were restimulated every 10 to 15 days in a 96-well plate (104/well) with irradiated autologous immortalized B cells pulsed with peptide (B-EBV, 4 × 104/mL well) and heterologous feeder cells (0.5-1 × 105/well) with PHA and rhIL-2. Control irrelevant CD4+ T-cell clones were expanded using the same procedure, except that the HIV peptides were omitted for restimulations.
ELISPOT assays
DCs or immortalized B cells were exposed for 2 to 3 hours to the indicated viruses (25-500 ng/mL or 0.5-10 nM p24 per 1.2 × 106 cells). When stated, AZT (5 μM; Sigma) was added to the cells 1 hour prior to viral exposure and maintained throughout the assay. Stimulator cells were then cocultured for at least 8 hours with CD4+ T-cell clones. IFN-γ production was measured in an enzyme-linked immunosorbent spot (ELISPOT) assay as described.21 As a positive control, stimulators were incubated with cognate peptides (at the indicated concentration) before addition of CD4+ clones. When stated, anti-DC-SIGN mAbs (20 μg/mL, 1B10 mAb [a kind gift from Ali Amara, Pasteur Institute, Paris, France] or 120507 mAb [R&D]), anti-CD4 mAb (Q4120, 20 μg/mL; a kind gift from Quentin Sattentau, Sir William Dunn School of Pathology, University of Oxford, United Kingdom), or chloroquine (Sigma) was added to stimulator cells 30 minutes prior to viral exposure. In experiments with chloroquine, stimulator cells were pulsed with viruses or peptides for 2 hours and cultured for an additional 6 hours in the presence of inhibitor only. Cells were then fixed (0.5% PFA for 1 minute at 4°C) and added to HS cells.
Intracellular cytokine and surface molecule stainings
Stimulators (B cells or DCs) were exposed for 2 to 3 hours to the indicated virus, washed twice, and incubated for 6 hours with effector T-cell clones at a 1:3 ratio (1 DC/3 T). With cell-free virion experiments, clones were directly exposed to HIVNL-AD8 (400 ng/mL or 8 nM p24), washed, and seeded in 96-well plates. As positive controls, stimulator cells were incubated with cognate peptides (at the indicated concentration) and effector cells treated either with PHA (1 μg/mL) or PMA (50 ng/mL; Sigma) in combination with a calcium ionophore (calcimycin, 1 μg/mL; Calbiochem, La Jolla, CA). When stated, cells were labeled with CFSE (2.5 μM; Molecular Probes, Eugene, OR) for 10 minutes at 37°C. Brefeldine-A1 (BFA, 5 μg/mL; Sigma) was added 5 hours before stainings. Cell-surface stainings were performed at 4°C for 30 minutes using anti-CD4 (13B8.2-APC; Beckman-Coulter, Hialeah, FL), anti-CD3 (SP34-2-PerCP; BD-Pharmingen, San Diego, CA), or anti-DC-SIGN (120507-APC/-PE; R&D) mAbs. Cytokine production was then detected using intracellular staining. Briefly, cells were fixed (4% PFA, 10 minutes), washed, and permeabilized (PBS, 0.1% BSA, 0.05% saponin, 15 minutes) prior to mAb staining. The following antibodies were used: anti-IL-2 (MQ1-17H12-PE), anti-IL-4 (MP4-25D2-PE), anti-IFNγ (B27-PE), and anti-TNFα (11-{9i}) (all from BD-Pharmingen). Anti-HIV-Gag (KC57-FITC; Beckman-Coulter) mAb was used to detect infected cells. Isotype-matched mAbs were used as negative controls. Samples were analyzed by flow cytometry using a FacsCalibur, with CellQuest software (both from Becton Dickinson, Lincoln Park, NJ).
HIV infection
Analysis of HS cell activation and HIV transmission by DCs. DCs were exposed to HIVNL-AD8 (40 or 400 ng/mL [0.8 or 8 nM, respectively]) p24) for 2 to 3 hours at 37°C, washed, and seeded in 96-well plates with T-cell clones at a 1:3 ratio. As positive controls, T cells were activated with PHA (1 μg/mL) for 2 hours at 37°C before coculture with DCs and maintained with rhIL-2. At the indicated time points, cells were analyzed by flow cytometry.
Analysis of HIV transmission by DCs to HS and irrelevant clones. DCs were exposed to HIVNL-AD8 at a low viral inoculum (1 or 10 ng/mL [0.02 or 0.2 nM, respectively] p24) for 2 to 3 hours at 37°C, washed, and seeded in 96-well plates with T-cell clones at a 1:1 ratio. For experiments without DCs, T-cell clones were similarly infected with cell-free virus, seeded in 96-well plates with rhIL-2. HIV content in supernatants was measured using Gag-p24 enzyme-linked immunosorbent assay (ELISA; NEN, Perkin-Elmer Life Sciences, Boston, MA).
When stated, CD4+ clones were activated by PHA and grown with rhIL-2 for 7 days. Cells were then infected for 2 to 3 hours at 37°C with HIVNL-AD8 using a high viral dose (100 ng/mL, 2 nM p24) with DEAE-dextran (10 μg/mL; Sigma). Viral replication was then followed using either p24 intracellular stainings or ELISA.
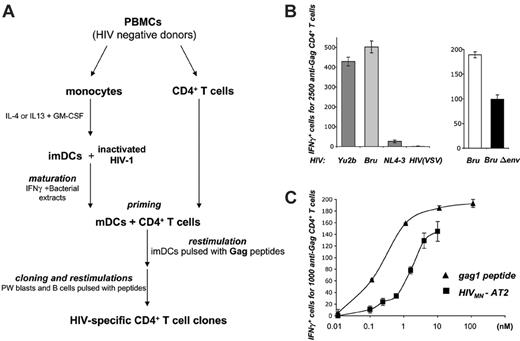
DCs present HIV-1 virion-derived antigens to HIV-specific (HS) CD4+ cells. (A) Protocol for generating HS CD4+ T-cell clones. Primary CD4+ T cells were primed with autologous monocyte-derived DCs pulsed with HIVMN virions inactivated with aldrilthiol-2 (HIVMN-AT-2). Cultures were restimulated with autologous DCs, pokeweed-stimulated (PW) blasts, or B-EBV cells pulsed with a pool of HIV-1 Gag-p24 peptides. Peptide-specific bulk cultures were then selected and HS clones derived by limiting dilution. (B) DCs exposed to HIV particles activate HS CD4+ cells. HLA-DRβ*01+ imDCs were exposed to the indicated HIV-1 strains in the presence of AZT, and cocultivated with IV9 HS cells for about 8 hours. Activation of IV9 cells was assessed in an IFNγ ELISPOT assay. Results are depicted as the number of IFNγ+ cells for the indicated number of effectors. Left panel: the indicated viral strains (5.4 nM p24) were used. YU2b (R5 tropic) or Bru (X4 tropic) carry the gag1 epitope recognized by IV9 cells, whereas this sequence is naturally mutated in NL4-3 strain (X4 tropic) and HIV(VSV) pseudotypes. Right panel: HIV Env increases MHC-II HIV antigen presentation by DCs. DCs were exposed to wild-type HIV Bru or Env-deleted (BruΔenv) isogenic viruses (0.5 nM p24). (C) Dose-response analysis of HIV antigen presentation by DCs. DCs were exposed to increasing concentrations of HIVMN-AT2 or, as a control, gag1 peptide, and tested as in panel B. For each panel, data are mean ± SD of triplicates and are representative of 3 independent experiments.
DCs present HIV-1 virion-derived antigens to HIV-specific (HS) CD4+ cells. (A) Protocol for generating HS CD4+ T-cell clones. Primary CD4+ T cells were primed with autologous monocyte-derived DCs pulsed with HIVMN virions inactivated with aldrilthiol-2 (HIVMN-AT-2). Cultures were restimulated with autologous DCs, pokeweed-stimulated (PW) blasts, or B-EBV cells pulsed with a pool of HIV-1 Gag-p24 peptides. Peptide-specific bulk cultures were then selected and HS clones derived by limiting dilution. (B) DCs exposed to HIV particles activate HS CD4+ cells. HLA-DRβ*01+ imDCs were exposed to the indicated HIV-1 strains in the presence of AZT, and cocultivated with IV9 HS cells for about 8 hours. Activation of IV9 cells was assessed in an IFNγ ELISPOT assay. Results are depicted as the number of IFNγ+ cells for the indicated number of effectors. Left panel: the indicated viral strains (5.4 nM p24) were used. YU2b (R5 tropic) or Bru (X4 tropic) carry the gag1 epitope recognized by IV9 cells, whereas this sequence is naturally mutated in NL4-3 strain (X4 tropic) and HIV(VSV) pseudotypes. Right panel: HIV Env increases MHC-II HIV antigen presentation by DCs. DCs were exposed to wild-type HIV Bru or Env-deleted (BruΔenv) isogenic viruses (0.5 nM p24). (C) Dose-response analysis of HIV antigen presentation by DCs. DCs were exposed to increasing concentrations of HIVMN-AT2 or, as a control, gag1 peptide, and tested as in panel B. For each panel, data are mean ± SD of triplicates and are representative of 3 independent experiments.
Results
Generation of HIV-1-specific (HS) CD4+ T-cell clones
To study HIV antigen processing and presentation by MHC-II molecules, we first generated a panel of HS CD4+ T-cell clones, following a procedure outlined in Figure 1A. Primary CD4+ lymphocytes from 2 HIV-seronegative donors were primed in vitro with autologous, HIV-pulsed DCs. To avoid productive infection of the various cells in culture, monocyte-derived imDCs were exposed to virions inactivated with aldrilthiol-2 (AT-2).37 For T-cell priming, DCs were matured and then mixed with purified autologous CD4+ lymphocytes. Cultures were periodically restimulated with autologous DCs or B lymphocytes pulsed with a pool of HIV-1 Gag-p24 peptides. Five CD4+ T-cell clones were selected by limiting dilution. The specificity of the clones was determined by testing their responses to each individual peptide from the pool. Upon exposure to the cognate peptide (gag1 or gag2), the clones proliferated and secreted various cytokines such as IFNγ, TNFα, IL-2, and IL-4 (Table 1). This pattern of cytokine secretion may correspond to Th0 cells, a subset of T cells characterized by the secretion of both Th1 and Th2 cytokines.39 As controls, we also generated from the same donors CD4+ clones with unrelated antigenic specificity, which secrete cytokines upon activation by PHA or PMA/calcimycin (Table 1 and not shown).
Characteristics of CD4+ T-cell clones
. | . | . | Proliferation,*H3 uptake . | Cytokine secretion* . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Donor/name . | Restriction . | Specificity . | . | IFNγ . | TNFα . | IL-2 . | IL-4 . | |||
| HIV-specific CD4+ T-cell clones | ||||||||||
| BRE | ||||||||||
| IV-9 | HLA-DRβ*01 | gag1 | + | + | + | + | + | |||
| 420 | ||||||||||
| L11 | HLA-DRβ*04 | gag2 | + | + | + | + | + | |||
| N2 | HLA-DRβ*04 | gag2 | + | + | + | + | ND | |||
| N12 | HLA-DRβ*04 | gag2 | + | + | + | + | ND | |||
| F12 | HLA-DRβ*01 | gag2 | + | + | + | + | + | |||
| Control CD4+ T-cell clones | ||||||||||
| 420 | ||||||||||
| 2.2 | Unknown | Unknown | ND | – | – | – | ND | |||
| 3.8 | Unknown | Unknown | ND | – | – | – | ND | |||
. | . | . | Proliferation,*H3 uptake . | Cytokine secretion* . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Donor/name . | Restriction . | Specificity . | . | IFNγ . | TNFα . | IL-2 . | IL-4 . | |||
| HIV-specific CD4+ T-cell clones | ||||||||||
| BRE | ||||||||||
| IV-9 | HLA-DRβ*01 | gag1 | + | + | + | + | + | |||
| 420 | ||||||||||
| L11 | HLA-DRβ*04 | gag2 | + | + | + | + | + | |||
| N2 | HLA-DRβ*04 | gag2 | + | + | + | + | ND | |||
| N12 | HLA-DRβ*04 | gag2 | + | + | + | + | ND | |||
| F12 | HLA-DRβ*01 | gag2 | + | + | + | + | + | |||
| Control CD4+ T-cell clones | ||||||||||
| 420 | ||||||||||
| 2.2 | Unknown | Unknown | ND | – | – | – | ND | |||
| 3.8 | Unknown | Unknown | ND | – | – | – | ND | |||
+ indicates positive; –, negative; ND, not done
Upon gag peptide stimulation. All clones secrete cytokines upon PMA/calcimycin stimulation (not shown). gag1 and gag2 correspond to Gag-p24(331-350) and Gag-p24(271-290) amino acid sequences, respectively
DCs present antigens derived from HIV-1 virions to HS cells
We asked whether these HS clones were activated by coculture with HLA-matched DCs that have been exposed to HIV-1 virions. We first studied IV9 cells, which recognize the HLA-DRβ*01-restricted gag1 peptide. To ensure that any presented antigens were derived from the viral input and not from newly synthesized proteins, HLA-DRβ*01+ imDCs were either pretreated with the reverse-transcriptase inhibitor AZT or exposed to AT-2-inactivated virions. DCs were pulsed with either YU2b (R5 tropic), Bru, or MN strains (X4 tropic), which all carry the gag1 sequence, or, as controls, with NL4-3 strain or HIV(VSV) pseudotypes, in which this sequence is mutated. Only HIV-1 strains carrying the cognate epitope allowed activation of anti-Gag CD4+ T cells, as measured in an IFNγ-ELISPOT assay (Figure 1B). Of note, IV9 cells were not activated when HLA-DRβ*01-negative cells were used as stimulators, indicating that this process is appropriately MHC-II restricted (not shown). Similar results were observed with L11 and N2 clones, which were activated upon coculture with HIV-pulsed autologous antigen-presenting cells (APCs; see “DC-SIGN promotes MHC-II-restricted HIV antigen presentation” and “DCs promote the activation and subsequent infection of HIV-specific CD4+ cells”).
When DCs were exposed to increasing concentrations of HIV MN-AT2, activation of IV9 cells appeared dose dependent (Figure 1C). The lowest effective viral input was about 10 ng/mL (or 0.2 nM) p24, and the half-maximun response was around 2 nM, which is only 10-fold higher than that observed with the synthetic gag1 peptide (0.2 nM) (Figure 1C).
DCs capture antigens by various means, including fluid-phase macropinocytic uptake and receptor-dependent internalization.7 We thus studied the role of HIV-1 envelope glycoproteins in MHC-II-restricted presentation of Gag epitopes. We examined the ability of DCs exposed to WT or Env-deleted (HIVΔenv) isogenic viruses to activate IV9 cells. We observed a 50% decrease of IFNγ-expressing cells with HIVΔenv, when compared with Env+ virions (Figure 1B).
Altogether, these results demonstrate that DCs present antigens derived from incoming virions to HS lymphocytes. This is an efficient process, observed with a viral inoculum in the nanomolar range, which corresponds to an input of 10 to 100 virions per DC. Moreover, both Env-dependent and -independent pathways of capture led to MHC-II antigen presentation in DCs.
DC-SIGN promotes MHC-II-restricted HIV antigen presentation
We next examined whether the lectin DC-SIGN, which binds HIV-1 Env with a high affinity,12 promotes MHC-II-restricted HIV antigen presentation. To this end, we derived EBV-immortalized B cells stably expressing DC-SIGN or not. We used B cells from 2 donors, corresponding to clones IV9 (B-BRE and B-BRE-DCS cells) and L11 (B-420 and B-420-DCS cells). B-BRE-DCS and B-420-DCS express surface DC-SIGN levels in the range of those observed on imDCs (Moris et al28 ). We first compared the ability of B-BRE and B-BRE-DCS to activate IV9 cells, upon exposure to increasing doses of HIVMN-AT2. Whatever the viral input, we observed a 2- to 3-fold increase in the number of IFNγ-expressing IV9 cells when DC-SIGN was present (Figure 2A). In contrast, the lectin did not influence presentation of the synthetic gag1 peptide (not shown). Facilitation of HIV antigen presentation was also observed with B-420-DCS cells and the CD4 T-cell clone L11 (Figure 2B). Using both R5 (YU2b) and X4 (MN and Bru) tropic HIV strains, we observed a strong enhancement of L11 activation (4- to 15-fold increase of IFNγ-expressing cells) in the presence of the lectin, whereas the peptide gag2 was similarly presented by B-420 and B-420-DCS cells (Figure 2B).
CD4+ T cells secrete various cytokines (Table 1). We assessed activation of L11 cells by measuring TNFα production by flow cytometry, instead of the IFNγ-ELISPOT assay. B-420 and B-420-DCS were incubated with the gag2 peptide, or exposed to varying concentrations of HIVMN-AT2, and cocultivated with L11 cells. A double staining was then performed (surface CD4 and intracellular TNFα) to distinguish T cells (CD4+) from B cells (CD4-). The gag2 peptide induced TNFα production by T cells (and not by B cells), without any notable difference when the lectin was present (Figure 2C). In contrast, with HIV, the percentage of TNFα+ T cells was increased 2- to 5-fold with DC-SIGN, whatever the viral input (Figure 2C). Similar results were obtained when measuring other cytokines (IL-2 or IFNγ; not shown).
DC-SIGN promotes MHC-II-restricted HIV antigen presentation. (A) Reactivity of HS clone IV9. B-BRE and B-BRE-DCS cells were used as stimulators in an IFNγ ELISPOT assay. The effectors were autologous IV9 HS cells that recognize the gag1 epitope. Stimulating cells were exposed to the indicated concentrations of HIVMN-AT2. (B) Reactivity of HS clone L11. B-420 and B-420-DCS cells were used as stimulator cells in an IFNγ ELISPOT assay. The effectors were autologous L11 HS cells that recognize the gag2 epitope. B cells were exposed to HIVMN-AT2, HIVBru (both at 10 nM p24), and HIVYu2b (4 nM p24), or to the cognate gag2 peptide (100 nM). (A-B) Data are mean ± SD of triplicates and are representative of 3 independent experiments. (C) Analysis of the role of DC-SIGN by flow cytometry. B-420 and B-420-DCS cells were used as stimulators in an intracellular cytokine assay measuring the activity of autologous L11 HS cells. Stimulating cells were exposed to HIVMN-AT2 or to the cognate gag2 peptide at the indicated concentrations. After 6 hours of coculture, cells were stained for surface CD4 and intracellular TNFα, and analyzed by flow cytometry. Numbers in the right corner indicate the percentage of TNFα-positive cells among L11 cells, which are CD4+. B-420 and B-420-DCS cells are CD4-. Isotypic mAbs (Ig) were used as a negative control. Data are representative of 3 independent experiments.
DC-SIGN promotes MHC-II-restricted HIV antigen presentation. (A) Reactivity of HS clone IV9. B-BRE and B-BRE-DCS cells were used as stimulators in an IFNγ ELISPOT assay. The effectors were autologous IV9 HS cells that recognize the gag1 epitope. Stimulating cells were exposed to the indicated concentrations of HIVMN-AT2. (B) Reactivity of HS clone L11. B-420 and B-420-DCS cells were used as stimulator cells in an IFNγ ELISPOT assay. The effectors were autologous L11 HS cells that recognize the gag2 epitope. B cells were exposed to HIVMN-AT2, HIVBru (both at 10 nM p24), and HIVYu2b (4 nM p24), or to the cognate gag2 peptide (100 nM). (A-B) Data are mean ± SD of triplicates and are representative of 3 independent experiments. (C) Analysis of the role of DC-SIGN by flow cytometry. B-420 and B-420-DCS cells were used as stimulators in an intracellular cytokine assay measuring the activity of autologous L11 HS cells. Stimulating cells were exposed to HIVMN-AT2 or to the cognate gag2 peptide at the indicated concentrations. After 6 hours of coculture, cells were stained for surface CD4 and intracellular TNFα, and analyzed by flow cytometry. Numbers in the right corner indicate the percentage of TNFα-positive cells among L11 cells, which are CD4+. B-420 and B-420-DCS cells are CD4-. Isotypic mAbs (Ig) were used as a negative control. Data are representative of 3 independent experiments.
Role of DC-SIGN and CD4 molecules in primary DCs. (A) Effects of anti-DC-SIGN and anti-CD4 mAbs on MHC-II-restricted HIV antigen presentation by B-cell lines. Stimulators (BRE and B-BRE-DCS cells) were exposed to HIVMN-AT2 (1 nM p24) for 2 hours at 37°C. Anti-DC-SIGN (1B10), anti-CD4 (Q4120), and IgG isotype control mAbs (20 μg/mL) were added 30 minutes prior to and maintained during viral exposure. Cells were then cocultivated with HS cells (clone IV9) for about 8 hours, and activation of IV9 cells was assessed in an IFNγ ELISPOT assay. (B) Role of DC-SIGN and CD4 molecules in primary imDCs. Stimulators (primary DCs) were treated with mAbs as described in panel A, and were then exposed to HIVMN-AT2 (1 nM p24) or to gag1 peptide (22 nM) and cocultivated with IV9 cells. Activity of IV9 cells was tested as in panel A. (C) Effects of anti-DC-SIGN mAbs on various HIV strains in primary imDCs. ImDCs (from a different donor than in B) were treated with mAbs as described in panel A, and were exposed to the indicated viruses: HIVYu2b, HIVMN-AT2 (produced in 293T and T1 cells, respectively, at 4 nM p24), and HIVBru (produced in activated PBMCs, 0.5 nM p24). Cells were then cocultivated with IV9 cells. Activity of IV9 cells was tested as in panel A. All experiments were performed in the presence of AZT. For each panel, data are mean ± SD of triplicates and are representative of 3 independent experiments.
Role of DC-SIGN and CD4 molecules in primary DCs. (A) Effects of anti-DC-SIGN and anti-CD4 mAbs on MHC-II-restricted HIV antigen presentation by B-cell lines. Stimulators (BRE and B-BRE-DCS cells) were exposed to HIVMN-AT2 (1 nM p24) for 2 hours at 37°C. Anti-DC-SIGN (1B10), anti-CD4 (Q4120), and IgG isotype control mAbs (20 μg/mL) were added 30 minutes prior to and maintained during viral exposure. Cells were then cocultivated with HS cells (clone IV9) for about 8 hours, and activation of IV9 cells was assessed in an IFNγ ELISPOT assay. (B) Role of DC-SIGN and CD4 molecules in primary imDCs. Stimulators (primary DCs) were treated with mAbs as described in panel A, and were then exposed to HIVMN-AT2 (1 nM p24) or to gag1 peptide (22 nM) and cocultivated with IV9 cells. Activity of IV9 cells was tested as in panel A. (C) Effects of anti-DC-SIGN mAbs on various HIV strains in primary imDCs. ImDCs (from a different donor than in B) were treated with mAbs as described in panel A, and were exposed to the indicated viruses: HIVYu2b, HIVMN-AT2 (produced in 293T and T1 cells, respectively, at 4 nM p24), and HIVBru (produced in activated PBMCs, 0.5 nM p24). Cells were then cocultivated with IV9 cells. Activity of IV9 cells was tested as in panel A. All experiments were performed in the presence of AZT. For each panel, data are mean ± SD of triplicates and are representative of 3 independent experiments.
Therefore, by using 2 HS clones having different specificities, we demonstrated that DC-SIGN expression by B cells facilitates MHC-II-restricted presentation of HIV virion antigens and enhances secretion of various cytokines by T cells.
Role of DC-SIGN and CD4 molecules in primary DCs
We then investigated the role of the lectin in DCs by using anti-DC-SIGN mAbs (1B10 [Figure 3] or 120507 [not shown]) known to block interactions between the lectin and gp120.28 We first verified that these mAbs were efficient in B cells. As expected, preincubation of B-BRE-DCS cells with mAbs (before viral exposure) potently inhibited HIVMN-AT2 antigen presentation and activation of IV9 cells (Figure 3A). Of interest, these mAbs were less efficient in DCs. Blocking DC-SIGN reduced by about 50% T-cell activation mediated by HIVMN-AT2-exposed DCs, without affecting presentation of the cognate peptide (at 22 nM [Figure 3B] or at lower concentrations [not shown]). The mAbs also decreased by 2-fold IFNγ secretion by IV9 cells, when using DCs from different HLA-DRβ*01+ donors, exposed to R5 (YU2b) and X4 (MN and BRU) viruses (Figure 3C). This level of inhibition is in line with our findings (Figure 1B) that HIV Env-mediated internalization accounts for half of the capture pathways leading to MHC-II presentation. Of note, in these experiments we used virions originated either from infected lymphoid cell lines or primary T cells, or from transfected 293T cells, and obtained similar results (Figure 3C and not shown), indicating that the type of virus-producing cells does not significantly affect MHC-II presentation.
We previously showed that the presence of a functional envelope on virions is required to induce MHC-I exogenous presentation of HIV antigens by DCs.21,28 Activation of anti-HIV CD8+ T cells by DCs was inhibited by neutralizing anti-CD4 mAb, and was no longer observed with virions bearing nonfusogenic envelope mutants.21,28 We thus examined whether gp120-CD4 interactions were involved in MHC-II presentation. Preincubation of DCs with the anti-CD4 mAb Q4120, which blocks HIV fusion and MHC-I antigen presentation (not shown and Moris et al28 ) did not affect activation of L11 cells by B-BRE-DCS cells or by DCs exposed to HIV (Figure 3A-B).
Therefore, DC-SIGN promotes MHC-II presentation of HIV antigens by DCs. The lectin is not the only means used by these cells to acquire virion antigens. HIV capture pathways leading to MHC-II presentation are different from those described for MHC-I epitopes.
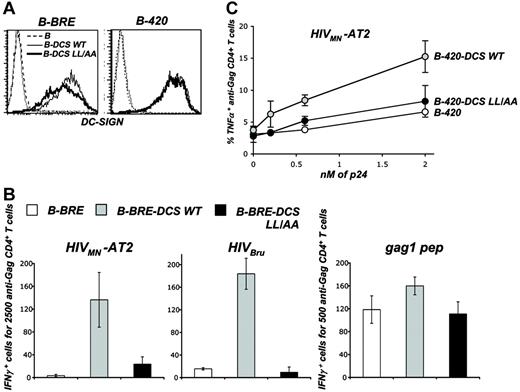
DC-SIGN trafficking and MHC-II-restricted HIV-1 antigen presentation. (A) Surface levels of DC-SIGN wild type (WT) and dileucine mutant (LL/AA) in B-cell lines. B-BRE cells (left panel) and B-420 cells (right panel) were transduced with a lentiviral vector coding for WT and LL/AA DC-SIGN, yielding B-BRE-DCS WT and B-420-DCS WT cells and B-BRE-DCS LL/AA and B-420-DCS LL/AA cells, respectively. In the latter, the dileucine sorting motif present in the cytoplasmic tail of DC-SIGN has been mutated. Cells were stained with anti-DC-SIGN Abs and analyzed by flow cytometry. An isotypic mAb was used as a negative control (dotted line). (B) Activity of B-BRE derivatives as stimulators of HS clone IV9. B-BRE, B-BRE-DCS WT, and B-BRE-DCS LL/AA cells were exposed to HIVMN-AT2, HIVBru (4 nM p24), or gag1 peptide (22 nM). Cells were then cocultivated with IV9 cells for 8 hours. Activity of IV9 cells was tested in an IFNγ ELISPOT assay. Data are mean ± SD of triplicates and are representative of 3 independent experiments. (C) Activity of B-420 derivatives as stimulators of HS clone L11. B-420, B-420-DCS WT, and B-420-DCS LL/AA cells were exposed to increasing concentrations HIVMN-AT2. Cells were then cocultivated with L11 cells for 6 hours. Activity of L11 cells was tested by measuring TNFα production by flow cytometry, as described in Figure 4. Results are presented as the percentage of TNFα-positive cells within CD4+ cells. Data are mean ± SD of duplicates and are representative of 3 independent experiments.
DC-SIGN trafficking and MHC-II-restricted HIV-1 antigen presentation. (A) Surface levels of DC-SIGN wild type (WT) and dileucine mutant (LL/AA) in B-cell lines. B-BRE cells (left panel) and B-420 cells (right panel) were transduced with a lentiviral vector coding for WT and LL/AA DC-SIGN, yielding B-BRE-DCS WT and B-420-DCS WT cells and B-BRE-DCS LL/AA and B-420-DCS LL/AA cells, respectively. In the latter, the dileucine sorting motif present in the cytoplasmic tail of DC-SIGN has been mutated. Cells were stained with anti-DC-SIGN Abs and analyzed by flow cytometry. An isotypic mAb was used as a negative control (dotted line). (B) Activity of B-BRE derivatives as stimulators of HS clone IV9. B-BRE, B-BRE-DCS WT, and B-BRE-DCS LL/AA cells were exposed to HIVMN-AT2, HIVBru (4 nM p24), or gag1 peptide (22 nM). Cells were then cocultivated with IV9 cells for 8 hours. Activity of IV9 cells was tested in an IFNγ ELISPOT assay. Data are mean ± SD of triplicates and are representative of 3 independent experiments. (C) Activity of B-420 derivatives as stimulators of HS clone L11. B-420, B-420-DCS WT, and B-420-DCS LL/AA cells were exposed to increasing concentrations HIVMN-AT2. Cells were then cocultivated with L11 cells for 6 hours. Activity of L11 cells was tested by measuring TNFα production by flow cytometry, as described in Figure 4. Results are presented as the percentage of TNFα-positive cells within CD4+ cells. Data are mean ± SD of duplicates and are representative of 3 independent experiments.
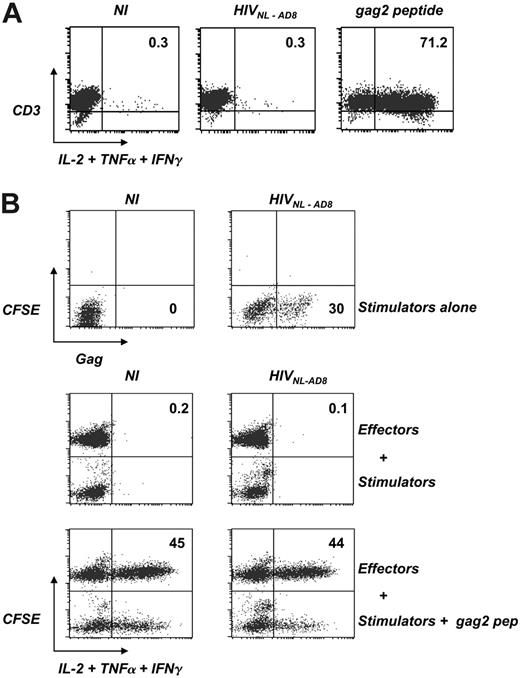
HS cells are not activated by cell-free virions or by HIV-infected lymphocytes. (A) Reactivity of HS clone L11 to cell-free virions. L11 cells were exposed to HIVNL-AD8 (8 nM p24) or the cognate gag2 peptide (44 nM) for 6 hours. Activity of L11 cells was tested by measuring intracellular cytokine production (IL-2, TNFα, and IFNγ) by flow cytometry. Cells were also stained with anti-CD3 mAbs. The percentage of cytokine+ cells is indicated. Isotypic mAbs were used as negative controls to set the quadrant position. Similar results were obtained after overnight incubation. Data are representative of at least 3 independent experiments. (B) Reactivity of HS clone L11 to HIV-infected lymphocytes. L11 cells previously activated by PHA and grown with IL-2 were infected with HIVNL-AD8 (8 nM p24). A few days later, the presence of productively infected cells was assessed by intracellular Gag staining (upper panel). The percentage of Gag+ cells is indicated. NI indicates control noninfected cells. Lower panels: These cells were then used as stimulators and cocultivated overnight with uninfected L11 cells, previously stained with CFSE. Cytokine production by stimulators (CFSE-) and effectors (CFSE+) was then assessed as described in panel A. As a positive control, stimulators were pulsed with the gag2 peptide (44 nM). The percentage of cytokine-positive cells among CFSE+ effectors is indicated. Data are representative of 3 independent experiments.
HS cells are not activated by cell-free virions or by HIV-infected lymphocytes. (A) Reactivity of HS clone L11 to cell-free virions. L11 cells were exposed to HIVNL-AD8 (8 nM p24) or the cognate gag2 peptide (44 nM) for 6 hours. Activity of L11 cells was tested by measuring intracellular cytokine production (IL-2, TNFα, and IFNγ) by flow cytometry. Cells were also stained with anti-CD3 mAbs. The percentage of cytokine+ cells is indicated. Isotypic mAbs were used as negative controls to set the quadrant position. Similar results were obtained after overnight incubation. Data are representative of at least 3 independent experiments. (B) Reactivity of HS clone L11 to HIV-infected lymphocytes. L11 cells previously activated by PHA and grown with IL-2 were infected with HIVNL-AD8 (8 nM p24). A few days later, the presence of productively infected cells was assessed by intracellular Gag staining (upper panel). The percentage of Gag+ cells is indicated. NI indicates control noninfected cells. Lower panels: These cells were then used as stimulators and cocultivated overnight with uninfected L11 cells, previously stained with CFSE. Cytokine production by stimulators (CFSE-) and effectors (CFSE+) was then assessed as described in panel A. As a positive control, stimulators were pulsed with the gag2 peptide (44 nM). The percentage of cytokine-positive cells among CFSE+ effectors is indicated. Data are representative of 3 independent experiments.
DC-SIGN trafficking and MHC-II-restricted HIV-1 antigen presentation
Trafficking of C-type lectins influences their capacity to deliver antigens to MHC loading compartments.40 It has been reported that upon binding of an anti-DC-SIGN mAb (that was used as a model antigen), mAb-lectin complexes are targeted to late endosomes/lysosomes and DC-SIGN ligands are then processed and presented by MHC-II.32 We previously demonstrated that DC-SIGN endocytosis is regulated by a dileucine (LL) motif in the cytoplasmic tail of the molecule.15,35 We thus examined the impact of DC-SIGN endocytosis on HIV-1 delivery to MHC-II loading compartments. To this end, we generated B-BRE and B-420 cells expressing a DC-SIGN LL/AA mutant and measured the capacity of the cells to activate IV9 and L11 clones, respectively. Surface levels of DC-SIGN WT and LL/AA were within the same range (Figure 4A). As expected,35 internalization of the lectin induced by anti-DC-SIGN mAb was impaired with the LL/AA mutant (not shown). B-BRE cells were pulsed with 2 HIV-1 isolates (MN-AT2 or BRU) and used to stimulate IV9 cells. DC-SIGN WT induced a strong activation of IV9 cells, whereas the LL/AA mutant was poorly efficient (Figure 4B). Presentation of gag1 peptide by B-BRE cells was similar in the presence of the 2 molecules (Figure 4B). B-420 derivatives were then pulsed with different concentrations of HIVMN-AT2 and used to stimulate L11 cells. Again, DC-SIGN WT, and not the LL/AA mutant, induced an efficient production of TNFα by L11 cells (Figure 4C).
Antigens presented by MHC-II are generally processed by acidic endosomal proteases.7 Incubation of HIV-pulsed DCs or B-BRE-DCS cells with chloroquine, a lysosomotropic weak base that neutralizes the acidic environment of endosomes, inhibited activation of IV9 cells in a dose-dependent manner (Figure S1, available at the Blood website; see the Supplemental Figures link at the top of the online article).
In conclusion, in B cells, mutation of the LL motif of DC-SIGN significantly reduces HIV-1 antigen presentation by MHC-II molecules. Both in DCs and DC-SIGN+ B cells, HIV antigens are degraded by acidic endosomal proteases. These results strongly suggest that DC-SIGN internalization routes HIV-1 antigen toward MHC-II loading compartments. Gag processing is then performed in an acidic endosomal compartment.
HIV-specific CD4+ cells are not activated by infected lymphocytes
We next asked whether other sources of viral antigens, such as cell-free virions and HIV-infected lymphocytes, might activate HS cells. Incubation of L11 cells with the cognate peptide (for 6 hours) induced a strong cytokine secretion, indicating that these cells were able to autopresent this peptide (Figure 5A). In contrast, HIV virions (NL-AD8 strain) did not activate L11 cells after 6 hours (Figure 5A) or longer incubation times (not shown). We then used HIV-infected L11 cells as stimulators. Cells were activated with PHA, grown with IL-2, and exposed to HIV NL-AD8 at a high multiplicity of infection (moi). A few days later, about 30% of the cells were productively infected, as assessed by intracellular Gag staining (Figure 5B). Infected cells were then cocultured with L11 cells labeled with CFSE. After an overnight incubation, neither CFSE+ nor CFSE- cells produced detectable levels of cytokines (Figure 5B). As a positive control, infected L11 cells were pulsed with the cognate peptide before overnight coculture with effectors. Under these conditions, both stimulator CFSE- cells, as well as CFSE+ cells, were efficiently activated (Figure 5B). This indicated that HIV-infected cells are not impaired in their ability to secrete cytokines. Of note, other HS clones (N2 and N12) behaved similarly and were not activated by coculture with infected lymphocytes (not shown).
Therefore, CD4+ lymphocytes, which are not professional APCs, do not present the gag2 epitope during productive HIV infection.
DCs promote the activation and subsequent infection of HIV-specific CD4+ cells
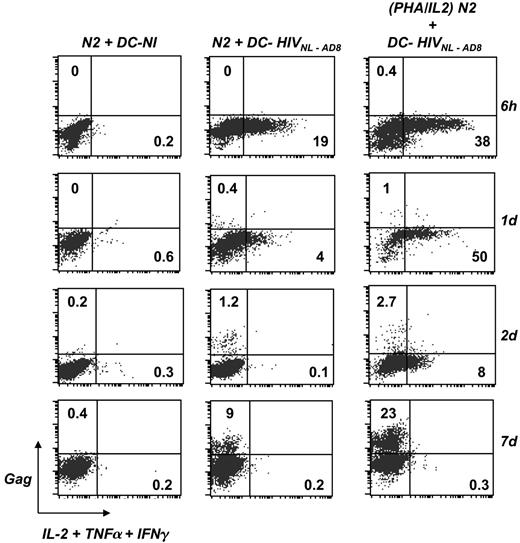
In vivo, HS CD4+ T cells are preferentially infected by HIV-1.1 We analyzed the links that may exist between T-cell activation by DCs and subsequent viral spread in lymphocytes. To address this point, DCs were exposed to HIV and then incubated with nonactivated HS N2 cells. At different time points of the coculture, from 6 hours to 7 days, lymphocytes were stained for cytokine production and Gag expression. To enhance detection of T-cell activation, a cocktail of anticytokine mAbs (TNFα, IL-2, IFN-γ) was used in these experiments. As expected, control noninfected DCs failed to activate N2 cells (Figure 6). In contrast, HIV-pulsed DCs induced a rapid and transient peak of cytokine production by N2 cells. In this representative experiment, at 6 hours after contact, 19% of the cells produced cytokines, and this percentage rapidly decreased to background levels at day 2. This transient activation was associated with a slow appearance of Gag+ lymphocytes. About 0.4% and 1.2% of HS lymphocytes were productively infected, at days 1 and 2 after contact, respectively. Viral replication spread and then reached 9% of Gag+ cells at day 7 (Figure 6). As a positive control, N2 cells were activated with PHA prior to incubation with DCs, and similarly analyzed. Activation was more robust with PHA, with 38% and 50% of the cells producing cytokines at 6 hours and day 1 after contact, respectively. Again, this activation was transient, and the fraction of cytokine+ cells decreased to 8% and 0.3% at days 2 and 7, respectively. This robust activation was associated with a higher viral replication rate, reaching 23% of Gag+ cells at day 7 (Figure 6). Similar results were observed with L11 and N12 cells, in which transient activation by HIV-pulsed DCs led to viral infection (not shown).
DCs promote activation and subsequent infection of HS CD4+ cells. imDCs were exposed to HIVNL-AD8 (0.8 nM of p24) for 3 hours at 37°C. Cells were then washed and cocultured with effector N2 cells that recognize the gag2 epitope, for the indicated periods of time. Noninfected DCs were used as a negative control (DC-NI). As a positive control for T-cell activation and HIV replication, N2 cells were activated with PHA for 2 hours prior to coculture with HIV-pulsed DCs. Cells were stained with anti-CD3, anti-DC-SIGN, anti-Gag, and anticytokine (IL-2, TNFα, and IFNγ) mAbs. N2 cell activation and HIV infection were analyzed by flow cytometry. Results depicted were obtained by gating the analysis on N2 cells (CD3+, DCSIGN-). The percentages of cytokine+ and Gag+ cells are shown. Data are representative of 3 independent experiments.
DCs promote activation and subsequent infection of HS CD4+ cells. imDCs were exposed to HIVNL-AD8 (0.8 nM of p24) for 3 hours at 37°C. Cells were then washed and cocultured with effector N2 cells that recognize the gag2 epitope, for the indicated periods of time. Noninfected DCs were used as a negative control (DC-NI). As a positive control for T-cell activation and HIV replication, N2 cells were activated with PHA for 2 hours prior to coculture with HIV-pulsed DCs. Cells were stained with anti-CD3, anti-DC-SIGN, anti-Gag, and anticytokine (IL-2, TNFα, and IFNγ) mAbs. N2 cell activation and HIV infection were analyzed by flow cytometry. Results depicted were obtained by gating the analysis on N2 cells (CD3+, DCSIGN-). The percentages of cytokine+ and Gag+ cells are shown. Data are representative of 3 independent experiments.
Therefore, DCs activate HS CD4+ lymphocytes and then successfully transfer HIV infection to T cells in the coculture. Increasing the activation state of HS cells with PHA facilitates further viral spread.
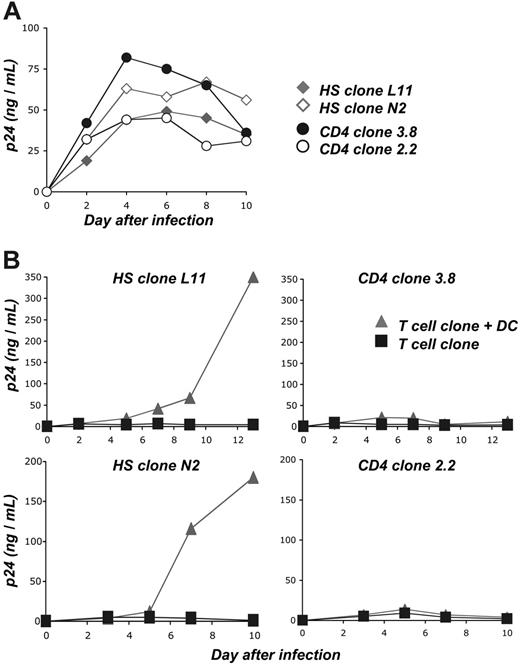
To further document these findings, we performed similar experiments with 2 HS clones (L11 and N2) and with 2 control CD4+ clones (2.2 and 3.8), and assessed viral replication by measuring p24 production in cell supernatants. The clones 3.8 and 2.2 are of unknown antigenic specifity. They do not recognize HIV-derived epitopes, but can be activated by PHA or by PMA/calcium ionophore treatment (Table 1; Figure S2). We first verified that these 4 clones were susceptible to HIV infection. Cells were thus treated with PHA, grown with IL-2, and exposed to HIV NL-AD8 at a high moi. Under this setting, viral replication occurred rapidly, without noticeable difference between the clones (Figure 7A). We then compared viral replication in nonactivated cells exposed to cell-free virions or cocultivated with autologous DCs pulsed with HIV at low moi (Figure 7B). With cell-free virions, viral replication was minimal, reaching 5 to 10 ng p24/mL at days 4 to 6 after infection in the 4 cell clones. These low levels likely corresponded to a residual replication in cells with reduced metabolic activity and/or limited proliferative capacities. The situation was different in the presence of DCs pulsed with low doses of HIV. With the 2 HS clones L11 and N2, a robust viral production occurred, reaching 200 to 350 ng p24/mL at day 13 after infection (Figure 7B). In contrast, HIV-pulsed DCs did not promote such an efficient viral replication in the 2 control clones. Viral replication peaked at 10 to 20 ng p24/mL (Figure 7B).
Altogether, these results suggest that the signals produced by HIV-exposed DCs trigger activation of HS cells and promote efficient viral replication in T cells. DCs minimally activate irrelevant CD4+ clones.
Discussion
We have studied the pathways of HIV antigen processing and presentation by MHC-II molecules in monocyte-derived DCs, the activation of HIV-specific (HS) CD4+ T cells, and the subsequent transfer of viral infection to these lymphocytes.
DCs promote infection of HS CD4+ cells. (A) Susceptibility of HS and control CD4+ clones to HIV replication. As a positive control for viral replication, 2 HS clones (L11 and N2) and 2 control clones with other antigenic specificities (3.8, 2.2) were activated with PHA and grown with IL-2. Cells were infected at a high moi (2 nM p24 for 3 × 105 cells). Viral replication was monitored at the indicated days by measuring Gag-p24 production in culture supernatants. (B) HIV-exposed DCs promote efficient viral replication in HS clones. imDCs were exposed to HIVNL-AD8 (0.2 nM p24 for 106 cells), washed, and cocultured with HS L11 or N2 cells and with control 3.8 or 2.2 cells. Cells were grown in the absence of exogenous IL-2. As a control, the 4 clones were directly exposed to the same viral inputs and cultured without DCs, in the presence of IL-2. Two independent experiments are assembled: autologous DCs were used in the upper panels, and HLA-matched (HLA-DRβ*04+) DCs were used in the lower panels. Viral replication was monitored by measuring p24 production in culture supernatants. Data are representative of 4 independent experiments.
DCs promote infection of HS CD4+ cells. (A) Susceptibility of HS and control CD4+ clones to HIV replication. As a positive control for viral replication, 2 HS clones (L11 and N2) and 2 control clones with other antigenic specificities (3.8, 2.2) were activated with PHA and grown with IL-2. Cells were infected at a high moi (2 nM p24 for 3 × 105 cells). Viral replication was monitored at the indicated days by measuring Gag-p24 production in culture supernatants. (B) HIV-exposed DCs promote efficient viral replication in HS clones. imDCs were exposed to HIVNL-AD8 (0.2 nM p24 for 106 cells), washed, and cocultured with HS L11 or N2 cells and with control 3.8 or 2.2 cells. Cells were grown in the absence of exogenous IL-2. As a control, the 4 clones were directly exposed to the same viral inputs and cultured without DCs, in the presence of IL-2. Two independent experiments are assembled: autologous DCs were used in the upper panels, and HLA-matched (HLA-DRβ*04+) DCs were used in the lower panels. Viral replication was monitored by measuring p24 production in culture supernatants. Data are representative of 4 independent experiments.
We generated a panel of HS CD4+ clones, recognizing 2 Gag-p24-derived epitopes, by performing an in vitro priming of naive PBMCs with autologous DCs pulsed with inactivated HIV particles. The use of cells from seronegative individuals, rather than from HIV-positive persons, avoided the presence of infected cells in the cultures and allowed us to obtain high amounts of functionally competent cells, which proliferated and secreted various cytokines in response to their cognate epitopes.
With this tool, we demonstrated that DCs capture, process, and present HIV antigens to CD4+ T cells, extending previous reports obtained with unsorted cells from HIV-positive individuals or from SIV-infected monkeys24-26 or using VSV-peudotyped HIV particules.41 We show here that MHC-II-restricted presentation of HIV antigens occurs at a low viral inoculum (in the nanomolar range), without requiring productive infection of DCs. Activation of HS cells was observed with fusion-competent HIV particles, and, to a lesser extent, with envelope-deleted virions. Therefore, various pathways of viral internalization lead to MHC-II presentation (eg, macropinocytosis and receptor-mediated capture). HIV Env-dependent pathways account for about 50% of antigen presentation, and are mediated mainly by the lectin DC-SIGN. However, DC-SIGN is probably not the only molecule involved in viral capture leading to MHC-II antigen presentation, and other receptors or lectins known to bind gp120, such as CD206,42 may also play a role in this phenomenon. Of importance, the role of DC-SIGN is not restricted to the binding of viral proteins. In B cells, mutating the LL endocytic signal in the cytoplasmic tail of DC-SIGN35 did not affect viral binding but significantly impaired antigen presentation. This suggests that DC-SIGN routes viral material toward MHC-II processing and loading compartments. Although DC-SIGN trafficking might be slightly different in B cells compared with DCs, our results are in line with our previous report that HIV particles captured by DCs and by DC-SIGN+ cells are rapidly degraded within endo/lysosomal compartments.28 Altogether, these results underscore the multiple destinies of incoming virions in DCs, and the intrinsic differences between MHC-I and MHC-II HIV antigen presentation. Both phenomena are promoted by DC-SIGN (this report and Moris et al28 ). MHC-I presentation is fusion dependent and requires proteasomal degradation of incoming virions in the cytosol,21,28 whereas MHC-II presentation occurs without fusion in acidic endosomal/lysosomal vesicles.
HIV productively infects macrophages and DCs. The Gag precursor carries sorting signals for multivesicular bodies (MVBs).43 Viral assembly occurs naturally in close proximity to the machinery of MHC-II presentation. It will be worth examining whether newly synthesized viral proteins represent a source of antigens for MHC-II. These HS CD4 clones will also be useful for studying other parameters of MHC-II antigen presentation, such as the influence of DC maturation, the effects of TLR ligands, and the potency of circulating blood DCs of different hematopoietic origins.
We show here that HS cells are activated by coculture with HIV-exposed DCs. Other sources of antigens such as autologous infected lymphocytes, and cell-free virions, failed to stimulate HS cells. In HIV-infected individuals, HS cells are functionally impaired. The origins of this phenomenon are not fully understood, but may include chronic contacts of HS cells with antigens, leading to immune exhaustion.2-5,44 Our results suggest that these signaling contacts are mostly mediated by APCs.
We also studied the links that may exist between activation and infection of HS lymphocytes. We show that HIV-exposed DCs deliver a signal that triggers infection of HS cells. Upon infection, HS clones did not continuously produce cytokines. Thus, the presence of HIV proteins, within CD4+ lymphocytes that normally recognize HIV-derived epitopes, does not induce cell activation or affect viral replication. In vivo, HIV-1 preferentially infects HS cells, through poorly defined mechanisms.1 Our results provide a mechanism explaining this preferred infection: we describe a dual effect of DCs, activating HS cells by presenting HIV epitopes and simultaneously spreading infection to these lymphocytes. HIV could spread from DCs to cytokine-positive or to bystander-negative HS cells in the coculture. We cannot discriminate between these 2 possibilities, which are not mutually exclusive. In any case, HS cell activation is prerequisite to HIV spreading. It has been recently demonstrated (in a mouse model) that an optimal T-cell differentiation process requires successive encounters with Ag-bearing DCs.45 These multiple contacts will likely increase the chances of successful cell activation and viral transfer.
Besides infecting HS cells, HIV mainly targets memory CD4+ T cells.1 The events described here may be operative with CD4 T cells with other antigenic specificities. Once activated by the relevant peptide, or by other stimuli, these memory T cells will be sensitive to HIV infection. In cell-culture experiments, this was demonstrated by using CMV epitopes as model antigens. In the presence of CMV antigens, HIV infection is preferentially transferred to CMV-specific cells within DC-T-cell clusters.6 In vivo, activation of CD4 memory T cells during opportunistic infection or after Bacille Calmette Guerin (BCG) vaccination may be associated with HIV replicative rebounds.46
Our observations have important implications regarding the design of anti-HIV vaccine strategies. Given the dual role of HS CD4 cells as immune effectors and targets for HIV infection, inducing a strong HS response may be deleterious for the host. An enhanced viral replication in vaccinated animals has been observed with various vaccine candidates against feline immunodeficiency virus (FIV) or simian immunodeficiency virus (SIV).47-49 Upon infection, an accelerated progression to AIDS has also been reported in one individual presenting vaccine-induced HS T-cell responses.50 Multiple mechanisms are likely involved in these accelerations. Although the majority (> 90%) of HS cells is not directly infected by HIV,1 this acceleration may be provoked by the vaccine-induced generation of virus-specific cells highly sensitive to infection. On the other hand, some efficacious vaccination protocols have been proposed. For instance, attenuated live SIV confers high levels of protection in macaques, through poorly understood mechanisms.51 In HIV-infected humans, the inoculation, as a therapeutic vaccine, of autologous DCs pulsed with AT-2-inactivated virions promotes some protective immunity.52 The HS clones described here provide a useful means to study various virologic and immunologic aspects of the interactions among HIV, DCs, and CD4+ T cells, and to assess in a cell-culture system the impact of vaccine candidates on T-cell activation and viral spread.
Prepublished online as Blood First Edition Paper, May 4, 2006; DOI 10.1182/blood-2006-02-006361.
Supported by grants from the Agence Nationale de Recherche sur le SIDA (ANRS), SIDACTION, the CNRS, the European Community, and Institut Pasteur. F.B. is a former fellow of European Community funding (LSHP-CT-2004-012169).
A.M. designed and performed experiments and wrote the paper; A.P., F.B., and F.G.-B. performed experiments; M.S. contributed vital reagents; and O.S. supervised the study and wrote the paper.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Nathalie Sol-Foulon for critical reading of the paper; Ali Amara, Florence Buseyne, Jeff Lifson, Quentin Sattentau, and the National Institutes of Health (NIH) AIDS research and Reference reagent program for the kind gift of reagents; and Nadège Bercovici, Jean-Pierre Abastado, and Yu-Chun Lone for discussions.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal