Abstract
We investigated immune reconstitution against human cytomegalovirus (HCMV) in 57 hematopoietic stem cell transplant (HSCT) recipients, aged 1 to 24 years, through a novel method combining T-cell stimulation by HCMV-infected autologous dendritic cells with simultaneous cytometric quantification of HCMV-specific, IFNγ-producing CD4+ and CD8+ T cells. Lymphoproliferative response (LPR) to HCMV antigens was also determined. Patients were stratified into 2 groups according to HCMV serostatus, comprising 39 HCMV-seropositive (R+) and 18 HCMV-seronegative (R–) patients who received a transplant from a sero-positive donor. Recovery of both HCMV-specific CD4+ and CD8+ T-cell immunity occurred in all 39 R+ patients within 6 months and in 6 (33%) of 18 R– patients within 12 months. In R+ patients, the median numbers of HCMV-specific CD8+ and CD4+T cells were significantly higher than those of healthy controls, starting from days +60 and +180, respectively. In R– patients, the median numbers of HCMV-specific T cells were consistently lower than in R+ patients. LPR was delayed compared with reconstitution of IFNγ-producing T cells. Patients with delayed specific immune reconstitution experienced recurrent episodes of HCMV infection. HCMV seropositivity of young HSCT recipients is the major factor responsible for HCMV-specific immune reconstitution, irrespective of donor serostatus, and measurement of HCMV-specific T cells appears useful for correct management of HCMV infection.
Introduction
Human cytomegalovirus (HCMV) infection and disease are still frequent complications of patients given a hematopoietic stem cell transplant (HSCT).1 Use of antiviral drugs, according to either the prophylactic or the preemptive therapy approach, has significantly reduced morbidity and mortality.2,3 However, both approaches have disadvantages, such as occurrence of late HCMV disease for prophylaxis4 and need of strict virologic monitoring for preemptive therapy.5
In humans, both HCMV-specific CD4+ and CD8+ arms of the T-cell immune response must be regenerated after HSC transplantation in order to obtain long-term protection against HCMV reactivation and disease.6,7 The identification of the immunodominant peptides of the 2 major antigenic viral proteins pp65 and p72,8,9 in combination with the use of HLA-peptide tetramer technology and flow cytometry, has allowed researchers to better define the kinetics and magnitude of peptide-specific CD8+ T-cell response during immune reconstitution.10,11 In addition, it was shown that CD4+ T cells are essential for sustained recovery of CD8+ T cells.12 While tetramer technology does not detect the functional activity of T cells, use of direct or autologous dendritic-cell (DC)–mediated stimulation of T cells by peptides deriving from pp65 and p72 in combination with cytokine flow cytometry (CFC) can identify the functional status of CD4+ and CD8+ T cells recognizing specific peptides.13,14 In addition, the introduction of the enzyme-linked immunospot (ELISPOT) assay in combination with HLA-peptide tetramer staining, as well as the direct coupling of epitopic peptide stimulation with tetramer staining, has improved the understanding of the protective activity of HCMV-specific T cells.15,16
Through a novel method17 based on the simultaneous quantification of HCMV-specific CD4+ and CD8+ T cells, we studied (1) virus-specific immune reconstitution in a population of young patients given an HSCT; (2) the comparative kinetics of recovery of IFNγ-producing CD4+ and CD8+ T cells, as well as the lymphoproliferative response (LPR) to HCMV antigens; (3) the correlation between occurrence of single or recurrent episodes of HCMV infection and virus-specific immune reconstitution; (4) the number of HCMV-specific CD4+ and CD8+ T cells critical for protection against HCMV infection; and (5) HSCT-related factors influencing immune reconstitution.
Patients, materials, and methods
Patients
From May 2003 through September 2005, a total of 61 patients (median age, 9 years; range, 1-24 years) undergoing allogeneic HSC transplantation were evaluated for HCMV-specific T-cell reconstitution. The characteristics of the 61 patients are reported in Table 1. Four seronegative patients (R–) receiving the graft from a seronegative donor (D–) were excluded from the analysis. The study protocol was approved by an institutional review board of the Istituto di Ricovero e Cura a Carattere Scientifico, Policlinico San Matteo, and patients or their parents provided informed consent in accordance with the Declaration of Helsinki.
Characteristics of the 61 patients analyzed
Characteristic . | Value* . |
|---|---|
| Sex, no. M/no. F patients | 32/29 |
| Median age at transplantation, y (range) | 9 (1-24) |
| Underlying disease | |
| Malignant | 37 (61) |
| Nonmalignant | 24 (39) |
| Stem-cell source | |
| Bone marrow | 43 (70) |
| Peripheral blood | 14 (23) |
| Cord blood | 4 (7) |
| Donor type | |
| Sibling | 22 (36) |
| Unrelated | 27 (44) |
| Haploidentical relative | 12 (20) |
| Donor/recipient HCMV serostatus | |
| D+/R+ | 28 (46) |
| D-/R+ | 11 (18) |
| D+/R- | 18 (29) |
| D-/R- | 4 (7) |
| Conditioning regimen | |
| TBI based | 27 (44) |
| Non-TBI based | 34 (56) |
| GvHD prophylaxis | |
| CS-A | 13 (21) |
| CS-A + MTX | 36 (59) |
| T-cell depletion | 12 (20) |
| Administration of ALG | |
| Yes | 27 (44) |
| No | 34 (56) |
| GvHD grade | |
| 0 to I | 44 (72) |
| II to IV | 17 (28) |
| Steroid therapy | |
| Yes | 26 (43) |
| No | 35 (57) |
Characteristic . | Value* . |
|---|---|
| Sex, no. M/no. F patients | 32/29 |
| Median age at transplantation, y (range) | 9 (1-24) |
| Underlying disease | |
| Malignant | 37 (61) |
| Nonmalignant | 24 (39) |
| Stem-cell source | |
| Bone marrow | 43 (70) |
| Peripheral blood | 14 (23) |
| Cord blood | 4 (7) |
| Donor type | |
| Sibling | 22 (36) |
| Unrelated | 27 (44) |
| Haploidentical relative | 12 (20) |
| Donor/recipient HCMV serostatus | |
| D+/R+ | 28 (46) |
| D-/R+ | 11 (18) |
| D+/R- | 18 (29) |
| D-/R- | 4 (7) |
| Conditioning regimen | |
| TBI based | 27 (44) |
| Non-TBI based | 34 (56) |
| GvHD prophylaxis | |
| CS-A | 13 (21) |
| CS-A + MTX | 36 (59) |
| T-cell depletion | 12 (20) |
| Administration of ALG | |
| Yes | 27 (44) |
| No | 34 (56) |
| GvHD grade | |
| 0 to I | 44 (72) |
| II to IV | 17 (28) |
| Steroid therapy | |
| Yes | 26 (43) |
| No | 35 (57) |
TBI indicates total body irradiation; CS-A, cyclosporine-A; MTX, methotrexate; ALG, antilymphocyte globulin; and GvHD, graft-versus-host disease.
Values are expressed as number of patients (percent of patients) unless otherwise indicated.
Virologic follow-up
HCMV infection/reactivation was considered detection by any assay of HCMV in blood in the absence of clinical manifestations or organ function abnormalities, while HCMV disease was defined as either systemic or local HCMV infection, associated with clinical symptoms and/or organ function abnormalities.18
Patients were monitored for HCMV reactivation for 6 to 12 months after HSCT; they were randomized to be assigned to monitoring of HCMV reactivation by either the antigenemia or the DNAemia assay, according to methods previously described.19,20 In the antigenemia arm, patients were treated upon first detection of either 2 or more pp65-positive leukocytes or upon first confirmed positivity, when a single positive cell was detected. Therapy was stopped upon 2 consecutive negative results. Relapse episodes were treated similarly.21 In the DNAemia arm, patients were treated when reaching a cutoff of 10 000 DNA copies/mL whole blood, while treatment was stopped after 2 consecutive tests in which less than 1000 DNA copies/mL were detected. Relapse episodes were treated similarly. Transient, self-resolving infection was defined by either a negative antigenemia test on the second control after a positive result or by repeated DNAemia values less than 10 000 DNA copies/mL.
In addition, viremia, as well as donor/recipient HCMV serostatus, was evaluated according to previously reported methods.22,23
Preemptive therapy of HCMV infection was based on administration of intravenous ganciclovir (5 mg/kg twice a day). Ganciclovir was replaced by foscarnet (90 mg/kg twice a day) in case of ganciclovir-induced neutropenia or increasing viremia levels.
Patient treatment
All patients were given a fully myeloablative preparative regimen. Graft-versus-host disease (GvHD) prophylaxis consisted of cyclosporine-A (Cs-A) alone or associated to a short course of methotrexate for patients receiving the allograft from an HLA-identical sibling, whereas patients who received a transplant from an unrelated donor received antilymphocyte globulin (ALG) before HSC transplantation in addition to Cs-A and short-term methotrexate. T-cell depletion of the graft was performed in patients given an HSCT from a haploidentical relative through positive selection of CD34+ G-CSF–mobilized peripheral-blood cells. Acute GvHD was treated with steroids as first-line therapy (methylprednisolone, 2 mg/kg per day), while patients with steroid-resistant disease were treated with extracorporeal photochemotherapy.24
Immunologic follow-up
HCMV-specific CD4+ and CD8+ T cells were simultaneously quantified by a novel method based on use of autologous, monocyte-derived, HCMV-infected immature DCs (iDCs) as previously described.17 Briefly, following in vitro generation from peripheral-blood mononuclear cells (PBMCs),25 iDCs were infected for 24 hours with an endotheliotropic and leukotropic strain of HCMV (VR1814), as previously reported.26,27 HCMV-infected iDCs were then cocultured overnight with autologous PBMCs at a ratio of 1:20 in the presence of 10 μg/mL brefeldin A (Sigma, St Louis, MO) to prevent cytokine release. Finally, PBMCs were tested for the frequency of HCMV-specific CD4+ and CD8+ interferon-γ (IFNγ)–producing T cells by the CFC assay.
Absolute CD3+CD4+ and CD3+CD8+ T-cell counts were determined on heparinized peripheral-blood samples by direct immunofluorescence flow cytometry (Beckman Coulter, Fullerton, CA). The total number of HCMV-specific CD4+ and CD8+ T cells was calculated by multiplying the percentages of HCMV-specific T cells positive for IFNγ by the relevant absolute CD4+ and CD8+ T-cell counts. Based on results obtained by testing a series of HCMV-seropositive and HCMV-seronegative healthy blood donors, “responders” (ie, patients in whom virus-specific immunity is present) were subjects with more than 0.4 HCMV-specific CD4+ or CD8+ T cells/μL blood.17
LPR against HCMV was measured as previously reported.28 Cutoff for LPR was considered as a stimulation index of 3.0 or more.
All patients were monitored for at least 6 months and 48 of them completed the 1-year follow-up. Of the remaining 13 patients, 2 died of transplant-related causes and 5 experienced leukemia relapse, while 6 are still in follow-up. Thirty-three HCMV-seropositive HSCT donors were taken as controls.
Statistical analysis
Data were analyzed as of September 15, 2005.
Patient-, disease-, and transplant-related variables were expressed as median and range or as percentage, as appropriate. The following variables were analyzed for their potential impact on HCMV-specific immune reconstitution: patient/donor age (the median was taken as cut point), T-cell depletion of the graft, ALG administration, occurrence of acute GvHD (0-I vs II-IV grades), need of steroid therapy, underlying disease (malignant vs nonmalignant disorders), conditioning regimen (total body irradiation-based vs chemotherapy-based), donor type (unrelated donor vs HLA-identical sibling), HCMV donor serostatus (D+ vs D–), stem-cell source (bone marrow vs peripheral blood), and type of monitoring (DNAemia vs antigenemia).
Differences between medians were compared by using the Mann-Whitney U test for unpaired data.
Curves of percentage of patients showing HCMV infection or HCMV-specific immune response in the first year after transplantation in the different groups of HSCT recipients were calculated and expressed as cumulative incidence, as reported,29,30 and compared by the log-rank test. P values lower than .05 were considered statistically significant. P values from .05 to .1 were considered statistically nonsignificant, but reported in detail. All variables with a P value less than .5 in univariate analysis were included in a multivariate analysis performed using the Cox-proportional hazard regression model. Receiver-operator curves (ROCs) analysis was performed to identify levels of HCMV-specific CD4+ and CD8+ T cells protective against HCMV infection.
Results
Incidence of HCMV infection and T-cell immune reconstitution in HCMV-seropositive and HCMV-seronegative HSCT recipients (R+ and R–)
None of the 4 D–/R– patients experienced HCMV infection, and, as expected, they did not show a specific immune response during the 12 months after transplantation.
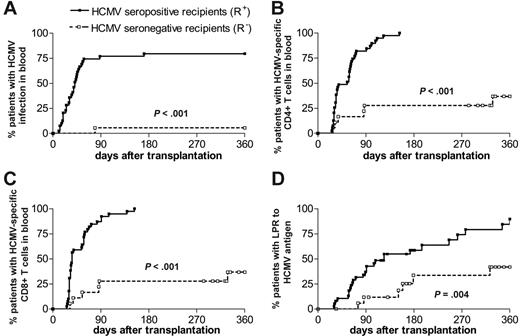
The cumulative incidence of HCMV infection/reactivation was significantly higher (P < .01) in R+ (n = 39) compared with R– (n = 18) patients during the first year after transplantation (Figure 1A). This higher incidence of virus reactivation was observed in R+ patients irrespective of the donor serologic status. Only 6 R+ patients developed viremia. No patient developed HCMV disease.
In parallel, the cumulative incidence curves show that a significantly higher number (P < .01) of R+ patients reconstituted (ie, showed a specific T-cell number > 0.4 cells/μL blood) both CD4+ (Figure 1B) and CD8+ (Figure 1C) HCMV-specific T cells compared with R– patients. More than 80% of R+ patients reconstituted HCMV-specific T cells within the first 3 months and all within 6 months after transplantation. By contrast, only 33% of R– patients developed specific immunity within the first year after transplantation, although their donor was HCMV seropositive.
A similar, although delayed, difference (P = .004) between R+ and R– patients was observed with regard to appearance of LPR to HCMV antigens (Figure 1D). In fact, only 60% of R+ patients reconstituted LPR within 6 months, while 15% of R+ patients were still unresponsive after 1 year.
HCMV infection and T-cell immunity reconstitution. Probability of developing an HCMV infection (A), of reconstituting HCMV-specific CD4+ (B) and CD8+ (C) T-cell immunity (ie, corresponding to a specific T-cell number > 0.4 cells/μL blood), and of restoring LPR to HCMV (D) in the 2 groups of HCMV-seropositive (R+) and -seronegative (R–) HSCT recipients.
HCMV infection and T-cell immunity reconstitution. Probability of developing an HCMV infection (A), of reconstituting HCMV-specific CD4+ (B) and CD8+ (C) T-cell immunity (ie, corresponding to a specific T-cell number > 0.4 cells/μL blood), and of restoring LPR to HCMV (D) in the 2 groups of HCMV-seropositive (R+) and -seronegative (R–) HSCT recipients.
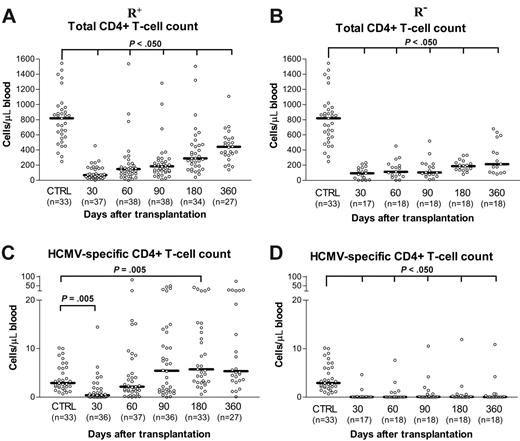
As shown in Figure 2A and B, the total CD4+ T-cell count increased over time after transplantation in both R+ and R– patients, respectively, although it remained significantly lower than that of controls. The total CD4+ T-cell count was comparable in R+ and R– patients at days +30 and +60, then becoming significantly higher (P < .05) in R+ patients from day +90 onward.
In the R+ group, the median number of HCMV-specific CD4+ T cells was lower than that of controls at day +30, comparable at day +60 and day +90, and significantly higher from day +180 onward (Figure 2C). The median number of HCMV-specific CD4+ T cells in the R– group of patients was consistently lower than those of both controls and R+ patients during the entire first year after transplantation (Figure 2D).
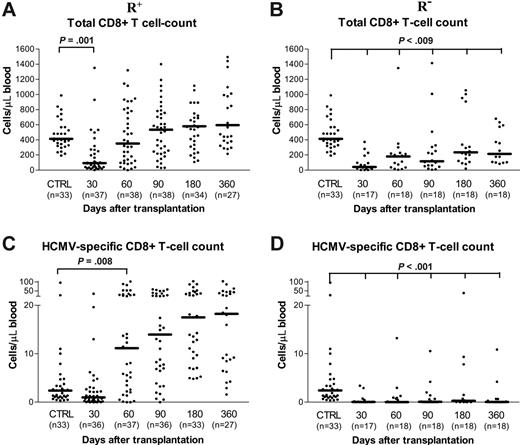
The total count of CD8+ T cells equaled that of controls at day +60 in R+ patients (Figure 3A), while in R– patients it remained lower than that of both controls and R+ patients for the entire follow-up period (Figure 3B). In R+ patients, the median number (Figure 3C) of virus-specific CD8+ T cells was significantly greater than that of controls, starting from day +60, with no significant further increase over time. By contrast, in R– patients, the median number of virus-specific CD8+ T cells was always significantly lower than that found in both R+ patients and controls (Figure 3D).
Factors influencing the kinetics of reconstitution of CD4+and CD8+ IFNγ-producing T cells
None of the factors analyzed influenced the recovery of HCMV-specific CD4+ immune response. Only the use of total body irradiation (TBI) positively correlated with better CD8+ HCMV-specific immune response at day +30, median time to virus-specific CD8+ T-cell detection being 34 days compared with 55 days for patients not receiving TBI (P = .021). This variable remained significant in multivariate analysis (data not shown). At the other time points, no variable influenced CD8+ HCMV-specific immune reconstitution.
Relationship of HCMV-specific T-cell reconstitution at day +60 and control of HCMV infection in R+ patients
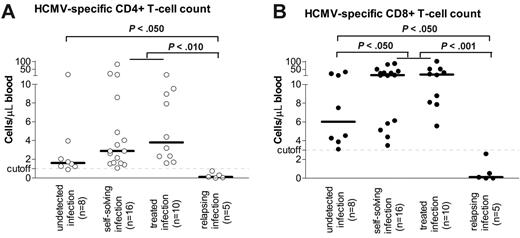
No difference in either HCMV-specific CD4+ or CD8+ T-cell counts, evaluated at day +60, was found between patients (n = 10) with HCMV infection resolving after a single antiviral drug course and patients (n = 16) experiencing a self-resolving infection, as defined before (Figure 4A-B). R+ patients with HCMV infection showed levels of virus-specific CD8+ T cells higher than those of patients (n = 8) with undetected infection (Figure 4B). Patients with relapsing episodes of HCMV infection (n = 5) requiring multiple treatment courses showed HCMV-specific CD4+ and CD8+ T-cell counts much lower than those of all other patients (Figure 4A and B, respectively).
Follow-up of total and HCMV-specific CD4+ T-cell reconstitution. Absolute number of total and of HCMV-specific CD4+ T cells during the first year after transplantation in the 2 groups of HCMV-seropositive (R+; A, C) and -seronegative (R–; B, D) recipients.
Follow-up of total and HCMV-specific CD4+ T-cell reconstitution. Absolute number of total and of HCMV-specific CD4+ T cells during the first year after transplantation in the 2 groups of HCMV-seropositive (R+; A, C) and -seronegative (R–; B, D) recipients.
The median absolute number of IFNγ-producing CD4+ and CD8+ T cells in 39 R+ patients at 60 days after transplantation is reported in Table 2. Numbers were stratified according to the occurrence of HCMV infection (undetected, self-resolving, requiring a single course of treatment, relapsing, and requiring multiple courses of treatment). Day +60 was chosen as it was the earliest time point permitting the identification of a threshold in the number of specific T cells conferring protection against recurrent HCMV reactivations. It can be speculated from our data that the emergence of 1 cell/μL for CD4+ and 3 cells/μL for CD8+ may confer protection against recurrent episodes of viral reactivation. Results of ROCs analysis for definition of optimal cutoffs for protection from recurrent infection are reported in Table 3.
Median absolute number of HCMV-specific T cells according to different types of HCMV infection in 39 R+ patients 60 days after transplantation
. | Median no. (range) of HCMV-specific T cells/μL . | . | |
|---|---|---|---|
| Type of HCMV infection . | CD4+ . | CD8+ . | |
| Undetected | 1.62 (0.91-15.78) | 6.03 (3.10-33.59) | |
| Self-solving | 2.88 (1.07-82.85) | 13.71 (3.49-88.18) | |
| Treated | 3.79 (1.60-15.01) | 17.26 (5.58-102.60) | |
| Relapsing | 0.12 (0.01-0.76) | 0.11 (0.01-2.60) | |
. | Median no. (range) of HCMV-specific T cells/μL . | . | |
|---|---|---|---|
| Type of HCMV infection . | CD4+ . | CD8+ . | |
| Undetected | 1.62 (0.91-15.78) | 6.03 (3.10-33.59) | |
| Self-solving | 2.88 (1.07-82.85) | 13.71 (3.49-88.18) | |
| Treated | 3.79 (1.60-15.01) | 17.26 (5.58-102.60) | |
| Relapsing | 0.12 (0.01-0.76) | 0.11 (0.01-2.60) | |
ROCs analysis for definition of optimal cutoffs for protection from recurrent infection
. | CD4+, % . | . | . | . | CD8+, % . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cutoff* . | Sens . | Spec . | PPV . | NPV . | Sens . | Spec . | PPV . | NPV . | ||||||
| 0.4 | 100 | 80 | 97 | 100 | 100 | 60 | 94 | 100 | ||||||
| 1.0 | 97 | 100 | 100 | 83 | 100 | 80 | 97 | 100 | ||||||
| 3.0 | 44 | 100 | 100 | 21 | 100 | 100 | 100 | 83 | ||||||
| 5.0 | 26 | 100 | 100 | 17 | 82 | 100 | 100 | 45 | ||||||
| 7.0 | 26 | 100 | 100 | 17 | 70 | 100 | 100 | 33 | ||||||
| 10.0 | 15 | 100 | 100 | 15 | 58 | 100 | 100 | 26 | ||||||
. | CD4+, % . | . | . | . | CD8+, % . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cutoff* . | Sens . | Spec . | PPV . | NPV . | Sens . | Spec . | PPV . | NPV . | ||||||
| 0.4 | 100 | 80 | 97 | 100 | 100 | 60 | 94 | 100 | ||||||
| 1.0 | 97 | 100 | 100 | 83 | 100 | 80 | 97 | 100 | ||||||
| 3.0 | 44 | 100 | 100 | 21 | 100 | 100 | 100 | 83 | ||||||
| 5.0 | 26 | 100 | 100 | 17 | 82 | 100 | 100 | 45 | ||||||
| 7.0 | 26 | 100 | 100 | 17 | 70 | 100 | 100 | 33 | ||||||
| 10.0 | 15 | 100 | 100 | 15 | 58 | 100 | 100 | 26 | ||||||
Sens indicates sensitivity; Spec, specificity; PPV, positive predictive value; and NPV, negative predictive value.
T cells per μL.
Follow-up of total and HCMV-specific CD8+ T-cell reconstitution. Absolute number of total and of HCMV-specific CD8+ T cells during the first year after transplantation in the 2 groups of HCMV-seropositive (R+; A, C) and -seronegative (R–; B, D) recipients.
Follow-up of total and HCMV-specific CD8+ T-cell reconstitution. Absolute number of total and of HCMV-specific CD8+ T cells during the first year after transplantation in the 2 groups of HCMV-seropositive (R+; A, C) and -seronegative (R–; B, D) recipients.
Discussion
We investigated the kinetics and magnitude of HCMV-specific T-cell immune reconstitution in young patients given an HSCT by means of a novel methodology.17 Our approach provides a more comprehensive assessment of T-cell reconstitution compared with methods exploiting stimuli given by single peptides or based on staining produced by tetramers, and has the following advantages: (1) its applicability is not limited by HLA restriction; (2) it takes advantage of the simultaneous expression on DC membrane of different viral proteins, including pp65 and p72; (3) it may be used for simultaneous quantification of both HCMV-specific CD4+ and CD8+ T cells by CFC; and (4) it allows a functional evaluation of T cells. The LPR assay complemented our approach in that it provided an estimate of the capacity of virus-specific T cells to proliferate in short-term cultures in response to viral antigens.
HCMV infection and HCMV-specific T-cell reconstitution. Relationship between absolute number of HCMV-specific CD4+ (A) and CD8+ (B) T cells and different types of HCMV infection at 60 days after transplantation in HCMV-seropositive HSCT recipients.
HCMV infection and HCMV-specific T-cell reconstitution. Relationship between absolute number of HCMV-specific CD4+ (A) and CD8+ (B) T cells and different types of HCMV infection at 60 days after transplantation in HCMV-seropositive HSCT recipients.
To our knowledge, this is one of the few prospective studies focusing on reconstitution of immune response against HCMV in pediatric patients given an HSCT. In our study, we found that all R+ patients recovered HCMV-specific CD4+ and CD8+ T-cell immunity within 6 months after transplantation, whereas R– patients, although receiving all HSCT from D+ donors, reconstituted HCMV-specific T-cell immunity in only 6 (33%) of 18 cases within 12 months. These data suggest that in this cohort of patients immune reconstitution was driven mainly by the pretransplantation HCMV infection of the recipient, in turn facilitating posttransplantation viral reactivation. This conclusion is in keeping with previous reports suggesting that HCMV infection of the recipient may act as a booster for donor-derived, antigen-experienced T cells.31,32 The relevance of virus-specific immune reconstitution sustained by donor T cells was recently emphasized in a large multicenter European study showing that R+ patients receiving graft from an unrelated D+ donor had an improved 5-year survival and a reduced transplant-related mortality (TRM) compared with R+ patients who received a transplant from a D– donor.33 Furthermore, seropositive patients who received a transplant from a D– donor have been reported to have an increased risk of developing high levels of antigenemia compared with R+ patients given the allograft from a D+ donor.34 In conclusion, in R+/D+ pairs, early immune recovery appears to be supported by adoptive transfer of memory T cells from donor to recipient, and HCMV infection may be crucial in favoring the expansion of memory T cells.
The observation that, in our cohort, patients receiving graft from a seronegative donor or a T-cell–depleted graft do not show a delay in HCMV-specific T-cell reconstitution raises the question of how a pathogen-specific immune response can develop in the absence of or after physical removal of memory T cells. Previously published studies in patients given a T-cell–depleted HSCT or a cord-blood transplant suggested that recipient-derived T-cell clones may also contribute to posttransplantation immune recovery.35,36 Thus, in some selected R+ patients early and sustained immune reconstitution may also be sustained by recipient T cells, whose survival and expansion is triggered by HCMV reactivation. Development of a primary immune response, although in the context of a profound state of immune deficiency characterizing the posttransplantation period, may be advocated to explain the presence of HCMV-specific T cells in R+ recipients who received a transplant from D– donors.37 In this regard, children may have a better capacity than adults in developing anti-HCMV primary immune response after HSC transplantation.
In R– patients who received a transplant from D+ donors, in the absence of detectable posttransplantation HCMV infection, immune reconstitution does occur only in a minority of patients. An antigen-independent, cytokine-driven expansion of donor memory T cells adoptively transferred with the graft can be taken into consideration for explaining immune reconstitution in these patients.38 Subclinical episodes of HCMV infection at sites other than blood may also have potentially contributed to the virus-specific immune reconstitution in R– patients.
Due to the very strict virologic monitoring and the early preemptive approach adopted, a few patients developed viremia and none suffered from HCMV disease. Thus, occurrence of relapsing infection requiring multiple courses of antiviral treatment was taken into account as an indicator of potentially severe HCMV infection. Results of ROCs analysis seem to confirm that levels of CD4+ less than 1 cell/μL and of CD8+ less than 3 cells/μL are not protective against recurrent infection. In our study, repeated episodes of HCMV reactivation occurred in patients without a prompt recovery of HCMV-specific immunity. Lack of posttransplantation immune regeneration has been reported to be consistently associated with relapses of HCMV infection and development of HCMV disease.4,12,14 In these cases, repeated courses of antiviral treatment are required to temporarily control viral infection, and this may cause emergence of drug-resistant HCMV strains or may lead to myelosuppression. Adoptive transfer of virus-specific T cells generated and expanded in vitro may be beneficial to these patients.39,40
In our patients reconstituting cellular immunity, the magnitude of HCMV-specific CD8+ T-cell reconstitution exceeds that of CD4+ T-cell response, the number of virus-specific CD8+ T cells being much higher than that found in controls and persisting during the entire follow-up period. This finding was already reported in previously published studies, showing in immunosuppressed individuals an increased presence of HCMV-specific CD8+ T cells that may represent up to 50% of the total CD8+ T-cell compartment, remaining elevated for months or even years.41
Our results support the previously published observation that when donors and recipients are HCMV seropositive, virus-specific CD4+ T-helper cells show the same reconstitution kinetics as CD8+ cytotoxic T cells.42 This finding is also in agreement with previous studies indicating that patients showing LPR to HCMV antigen (attributed to CD4+ T-cell activity) were protected against HCMV recurrent reactivation and disease.12 As already observed in primary HCMV infection of immunocompetent subjects and pregnant women,43 we found that recovery of LPR to HCMV antigens is delayed compared with reconstitution of CD4+ and CD8+ IFNγ-producing T cells.
While no factor influenced HCMV-specific CD4+ T-cell recovery, TBI as part of the preparative regimen was the only variable enhancing virus-specific CD8+ T-cell reconstitution at day +30. The interpretation of this finding remains unclear. It is possible that the relatively low number of patients analyzed precluded the possibility of identifying the impact of other variables.
In conclusion, our results demonstrate that many R+ young patients given an HSCT consistently reconstitute protective HCMV-specific immunity within 6 months. In the future, routine immunologic monitoring will be helpful in guiding virologic monitoring and therapeutic decisions in young HSCT recipients. Interventions with antiviral drugs could be limited in patients with T-cell immune response, while sustained lack of immune reconstitution could be compensated with interventions of adoptive T-cell transfer immunotherapy.
Prepublished online as Blood First Edition Paper, April 13, 2006; DOI 10.1182/blood-2005-11-012864.
Supported in part by grants from Ministero della Salute–IRCCS (Istituto di Ricovero e Cura a Carattere Scientifico) Policlinico San Matteo (Ricerca Corrente grants 80541 and 80425, and Ricerca Finalizzata 2003 grant 89269), Fondazione Cassa di Risparmio delle Provincie Lombarde (CARIPLO; grant 93005), AIRC (Associazione Italiana Ricerca sul Cancro), CNR (Consiglio Nazionale delle Ricerche), MURST (Ministero dell'Università e della Ricerca Scientifica e Tecnologica), and European Union (FP6 Program ALLOSTEM).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to M. Grazia Revello for helpful discussion, to Fausto Baldanti for HCMV real-time polymerase chain reaction (PCR) assay development, to Stefania Telli for data and sample collection, to Giuditta Comolli and Antonella Chiesa for flow cytometer analysis, to the entire technical staff of the Servizio di Virologia for performing the assays, and to D. Sartori for taking care of the article. We also thank Linda D'Arrigo for the revision of the English in this article.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal