Abstract
Communication between endothelial cells (ECs) and mural cells is critical in vascular maturation. Genetic studies suggest that angiopoietin/Tie2 signaling may play a role in the recruitment of pericytes or smooth muscle cells (SMCs) during vascular maturation. However, the molecular mechanism is unclear. We used microarray technology to analyze genes regulated by angiopoietin-1 (Ang1), an agonist ligand for Tie2, in endothelial cells (ECs). We observed that hepatocyte growth factor (HGF), a mediator of mural cell motility, was up-regulated by Ang1 stimulation. We confirmed this finding by Northern blot and Western blot analyses in cultured vascular endothelial cells. Furthermore, stimulation of ECs with Ang1 increased SMC migration toward endothelial cells in a coculture assay. Addition of a neutralizing anti-HGF antibody inhibited Ang1-induced SMC recruitment, indicating that the induction of SMC migration by Ang1 was caused by the increase of HGF. Interestingly, Ang2, an antagonist ligand of Tie2, inhibited Ang1-induced HGF production and Ang1-induced SMC migration. Finally, we showed that deletion of Tie2 in transgenic mouse reduced HGF production. Collectively, our data reveal a novel mechanism of Ang/Tie2 signaling in regulating vascular maturation and suggest that a delicate balance between Ang1 and Ang2 is critical in this process.
Introduction
Angiogenesis consists of the endothelial cell (EC) sprouting process and the vascular maturation process, which includes the recruitment of perivascular cells—smooth muscle cells (SMCs) for large vessels and pericytes for microvessels. In recent years, extensive efforts have been engaged in understanding the molecular mechanisms of angiogenesis. Several angiogenic growth factors that regulate the endothelial sprouting process have been identified. However, little attention has been focused on the vascular maturation process. The formation of a functional vasculature system is regulated by communications between ECs and SMCs.1,2 Interactions between these 2 cell types in the blood vessel wall have critical roles in the regulation of vascular formation, stabilization, remodeling, and function. Failure of the interactions between the 2 cell types, as seen in numerous genetic mouse models, results in severe and often lethal vascular defects.3 Therefore, a study of the molecular mechanisms of the vascular maturation process will enhance our understanding of angiogenesis and identify therapeutic targets for angiogenic diseases.
Angiopoietin/Tie2 signaling has been implicated in vascular maturation.4-6 Disruption of either Tie2 or its agonist ligand, angiopoietin 1 (Ang1), in transgenic mice causes embryonic lethality attributed to vascular defects characterized by reduced or absent SMC recruitment.4,6 Conversely, an activating mutation in Tie2 causes inherited venous malformation with abnormal SMCs on the blood vessel wall, suggesting that the Tie2 signaling pathway is critical for endothelial cell–smooth muscle cell communication in venous morphogenesis.7 In situ hybridization data revealed that Ang1 is mainly expressed in mature vessels. In contrast, the antagonist ligand of Tie2, Ang2, is primarily expressed in the growing vessels.5 It has been suggested that Ang2 destabilizes blood vessels during angiogenesis by dissociating SMCs from ECs and that Ang1 recruits SMCs and participates in vascular maturation.
Indeed, several studies have implicated Ang1 in the regulation of smooth muscle cell recruitment. Constitutive expression of Ang1 in lungs caused severe pulmonary hypertension because of the thickening of small pulmonary vessels from smooth muscle cell hyperplasia in rodents.8 A decrease in SMC dissociation from existing vessels and an increase in mesenchymal cell infiltration into tumor by Ang1 overexpression was observed in a mouse breast cancer model.9 Recently, a study10 showed that Ang1 up-regulates SMC recruitment through the induction of the heparin binding EGF-like growth factor (HB-EGF), which signals through ErbB1 and ErbB2 receptors. Taken together, it has been suggested that Ang1 stabilizes vessel development by stimulating the interactions between the endothelium and the periendothelium. Because SMC recruitment is essential for the structural and functional support of the endothelium, further understanding of the communication between these 2 cells is required.
Growth factors such as platelet-derived growth factor (PDGF), basic fibroblast growth factor-2 (bFGF), and transforming growth factor (TGF) regulate SMC migration.11 Hepatocyte growth factor (HGF), a mesenchyme-derived protein, has been implicated in a wide variety of cellular responses, including growth, cytoskeleton reorganization, and motility.12 Numerous types of cells, including endothelial cells, express HGF.13 The only known receptor for HGF, c-met, was also expressed by SMCs, suggesting that these cells can respond to HGF in vivo. Indeed, several studies have shown that HGF induces SMC migration,14-16 and potentially ERK1/2 signaling, by contributing to focal adhesion redistribution and to FAK and Pyk2 activation. In an in vivo model after balloon injury, HGF was reported to facilitate the migration of SMCs,16 and local administration of HGF was shown to accelerate reendothelialization and to attenuate neointimal proliferation.17
Here we studied the molecular mechanisms of angiopoietin-mediated SMC recruitment. We demonstrated that Ang1 and Ang2 have opposing effects on HGF production in cultured vascular endothelial cells that correlated with the recruitment of SMCs. We showed that Tie2 null mouse embryo, which exhibits defects in SMC recruitment, has a reduced HGF production. Our data also confirmed that yolk sac endothelial cells express HGF. Thus, our finding identifies a novel mechanism by which angiopoietins exhibited a “yin-yang” mechanism in regulating vascular maturation.
Materials and methods
Materials
Human umbilical vein endothelial cells (HUVECs) and human aortic smooth muscle cells (HASMCs) were purchased from Clonetics (San Diego, CA). HUVECs were grown on 0.1% gelatin-coated plates in endothelial growth medium (EGM; Clonetics) and were kept in a humidified incubator with 5% CO2 at 37°C. HUVEC passages 3 to 7 were used in this study. HASMCs were grown in M199 plus 15% FBS. Renal microvessel endothelial cells (RMECs) were kindly provided by Dr T. Takahashi (Vanderbilt University), and RMECs were grown in endothelial cell growth factor–supplemented DMEM.18 Mouse yolk sac endothelial cells (C166) were maintained as described.19
The adenoviral vectors directing the expression of Ang1* (AdAng1) and Ang2 (AdAng2) were provided by Dr George D. Yancopoulos (Regeneron, Tarrytown, NY). Ang1* is a slightly modified version of Ang1 that is easier to express and purify.20 Adenoviral vector directing the expression of murine VEGF165 (AdVEGF) was constructed as described.21 Adenoviral vectors directing the expression of a dominant-negative Akt1 (Addn-Akt) was provided by Dr Wataru Ogawa at Kobe University.22 An adenoviral vector directing the expression of a LacZ gene (Adβ-gal) was used as a viral vector control. Viral vectors were propagated in 293 cells and purified in a CsCl column as described.21
Northern blotting
Cells were grown in 10-cm culture plates to 70% confluence and then infected with adenoviral vectors that directed the expression of the gene of interest at an MOI of 10 for 16 hours, 24 hours, and 48 hours. In the experiment observing the negative effect of Ang2 against Ang1 in HGF mRNA up-regulation, media from AdAng1*, AdAng1* plus AdAng2, or Adβ-gal–infected cells after 24 hours of infection were fed to nontransfected HUVECs for the next 24 hours. RNA was isolated using the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. Total RNA (5 μg) was separated on a 1.2% agarose gel and transferred to a nylon membrane (Amersham Pharmacia Biotech, Piscataway, NJ). Full-length HGF cDNA was provided by Dr Yuhua Liu at the University of Pittsburgh, and a 32P-labeled probe for HGF mRNA was prepared with Prime-It Random Primer Labeling Kit (Stratagene, La Jolla, CA) and was hybridized with Express Hyb (Clontech, Palo Alto, CA). Band intensity of mRNA was quantified using a National Institutes of Health image 1.61/ppc and was normalized with 18S RNA.
Kinase assays
Akt kinase assay was conducted using an Akt kinase assay kit (Cell Signaling, Beverly, MA) according to the manufacturer's protocol. Briefly, HUVECs were infected with viral vectors that directed the gene of interest for 48 hours. Two hundred micrograms protein was mixed with 20 μL immobilized antibody bead slurry and rocked overnight at 4°C. Samples were centrifuged, washed, resuspended in kinase buffer with 1 μL of 10 mM ATP and 1 μg GSK-3 fusion protein, and incubated for 30 minutes at 30°C. The samples were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and blotted with primary antibody (1:1000, anti–phospho–GSK-3α/β) (Ser21/9 antibody) and secondary antibody (1:2000).
For ERK activation, HUVECs were treated with the ERK inhibitor PD98059 (Calbiochem, San Diego, CA) at 20 μM for 30 minutes before viral infection. As a positive control, cells were treated with PD98059 at 20 μM for 1 hour and then were treated with bFGF (100 ng/mL) for 30 minutes. Thirty micrograms total protein was separated by SDS-PAGE. The membrane was blotted with primary antibody (1:1000 mouse anti–phospho-ERK; Cell Signaling) and horseradish peroxidase–conjugated secondary antibody (1:10 000 antimouse antibody; Promega, Madison, WI). The membrane was developed using ECL Western blotting detection reagents (Perkin-Elmer Life Sciences, Boston, MA). The same membrane was stripped and reblotted with an ERK antibody (1:2000; Cell Signaling) for the detection of ERK protein levels. For p38 MAPK activation, HUVECs were treated with SB30580 (Calbiochem) at 10 μM for 30 minutes before viral infection.
Western blotting
For the detection of Ang1, Ang2, and VEGF expression in the viral vector–infected cells, conditioned media without serum were collected 24 hours after viral infection. The media were concentrated with a Centricone filter (Amicon, Beverly, MA) and then analyzed by Western blotting. Immunoblotting of conditioned media was performed with an anti-Ang1 antibody or an anti-Ang2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and a horseradish peroxidase–conjugated antimouse secondary antibody (Promega, Madison, WI) or an anti-VEGF antibody (Santa Cruz Biotechnology) and a horseradish peroxidase–conjugated antirabbit secondary antibody (Promega).
HGF protein was detected from RMEC cell lysates with an anti–human HGF antibody (R&D Systems, Minneapolis, MN); β-tubulin (Sigma, St Louis, MO) as a loading control and a horseradish peroxidase–conjugated antimouse secondary antibody (Promega) were used. Membranes were developed with the use of ECL Western blotting detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ).
Smooth muscle cell migration
A coculture migration assay was performed as described with some modifications.23 Briefly, on day 1, HUVECs were infected with various adenoviral vectors expressing the gene of interest. HASMCs were infected with an adenoviral vector expressing a red fluorescent protein (RFP) at an MOI of 20 for detection of the migrated SMCs. On day 2, 1 × 105 infected or noninfected HUVECs were plated on the underside of Transwells (Costar, Cambridge, MA) with 8-μm pore size filter chambers (VWR Scientific, West Chester, PA), which was coated with growth factor–depleted Matrigel (Sigma). After 2 hours of incubation, the filter was placed back into the 24-well plate. On day 3, HUVECs and HASMCs were serum starved overnight with DMEM. On day 4, 5 μg/mL HGF-neutralizing antibody (R&D Systems), 5 μg/mL control IgG (R&D Systems), or 50 ng/mL recombinant human HGF (R&D Systems) as a positive control was added to different wells and incubated for 1 hour at 37°C. After incubation, 2.5 × 104 HASMCs were added in the upper side of the filters and were incubated for 5 hours at 37°C. After incubation, cells on the upper side of the filters were removed with cotton-tipped swabs, and the red cells on the bottom of each filter were counted at 200 × field (5 fields/filter).
Real-time PCR analysis
Total RNA was prepared from yolk sac tissues of knockout or wild-type day 9.5 embryos and from yolk sac endothelial cells (C166). cDNA was synthesized from 1 μg total RNA with Thermoscript reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Specific primer sets for HGF (2 sets of primers) and GAPDH used for real-time RT-PCR analysis were: HGF forward, 5′ ACCAAGGAAG ACCCATTACTGAAGA; HGF reverse, 5′ TTCCAAGGCTGGCATTTGATGC; HGF forward, 5′ TGAGACTGATGTCCCTATGGAAAC; HGF reverse, 5′ AGTATCTCCTTCACAACGGGAAA; GAPDH forward, 5′ ACCACAGTCCATGCCATCAC; GAPDH reverse, 5′TCCACCACCCTGTTGCTGTA.
Quantitative real-time PCR was performed with 5 μL cDNA solution with Universal PCR Master Mix (Qiagen), SYBR-Green, and 200 nM primer on an ABI PRISM 7000 Sequence Detection System (GE Life Sciences, Fairfield, CT). Relative gene expression levels were normalized according to the expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and was calculated using the comparative Ct method, described in user bulletin 2 (ABI; GE Life Sciences). Expression data represent the average values from 3 different experiments.
Statistical analysis
Results are reported as mean ± SEM. Statistical analysis was performed with the 2-tailed Student t test. Differences were considered statistically significant at P < .05.
Results
Ang1 induced HGF expression in endothelial cells
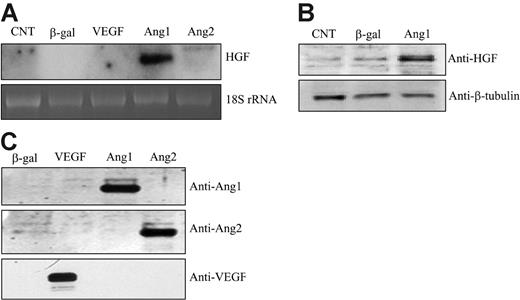
Ang/Tie2 signaling has been implicated in regulating the recruitment of SMCs during vascular maturation. However, the molecular mechanism is unclear. In searching for the gene that is responsible for Ang1-mediated SMC recruitment, we performed microarray analysis. HUVECs were infected with an adenoviral vector expressing Ang1 that enabled constant production of Ang1 or a control vector expressing LacZ, respectively, followed by microarray analysis. We found that HGF was dramatically up-regulated by Ang1 stimulation in cultured endothelial cells (data not shown). This finding was further confirmed by Northern blot analysis, which showed that Ang1 significantly induced HGF mRNA production in HUVECs (14.3- ± 4.5-fold) compared with β-gal controls (n = 10; P < .05) (Figure 1A). Increased HGF expression was also observed in human renal microvessel endothelial cells (RMECs; data not shown). Thus, gene induction was not a cell-specific finding. The time course study showed that HGF mRNA was induced at 16 hours, when the virally encoded Ang1 was expressed, and the level was sustained at least for 48 hours (data not shown). In contrast, we did not observe any significant HGF induction by other angiogenic factors, including VEGF and Ang2 (Figure 1A). In addition, cell lysates were collected 48 hours after viral infection and were subjected to Western blot analysis for HGF protein production. The result confirmed that the HGF protein level was also increased upon Ang1 stimulation in HUVECs (Figure 1B).
To ensure proper gene expression and Ang1 function, conditioned media were collected and analyzed by Western blotting for Ang1, Ang2, and VEGF expression in the supernatant of cultured cells. Each protein was easily detected in the culture media (Figure 1C).
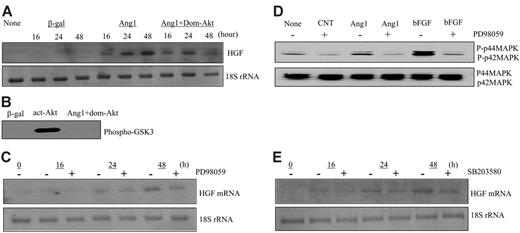
Studies have shown that Ang1 activates Akt, MAPK, and p38 MAPK in endothelial cells.24-28 To dissect the signaling pathway by which HGF was induced by Ang1, we used specific inhibitors to block the activation of these known downstream mediators—a specific inhibitor for MAPK/ERK, PD98059, a specific inhibitor for p38 MAPK, SB203580, and a dominant-negative Akt (Addom-Akt). For Akt inhibition, HUVECs were coinfected with AdAng1 or Addom-Akt. For MAPK and p38 MAPK inhibition, HUVECs were pretreated with PD98059 at 20 μM or with SB203580 at 10 μM, respectively, before Ang1 stimulation. Total cellular RNAs were isolated at 16, 24, and 48 hours after viral infection and were analyzed by Northern blotting. The data showed that neither AKT, MAPK, nor p38 MAPK was involved in HGF gene induction by Ang1 (Figure 2A, C, E). To ensure the proper function of these inhibitors, we harvested the cells and analyzed the cell lysate for Akt and MAPK activity, respectively. Inhibition of Akt activity by Addom-Akt was confirmed by an Akt kinase assay using GSK-3 fusion protein as a substrate (Figure 2B). In addition, PD98059 function was confirmed by the inhibition of phosphorylation of p42/p44 MAPK observed on Western blot (Figure 2D). Collectively, the known mediators of Tie2 signaling did not participate in Ang1-induced HGF expression, indicating that other signaling mediators downstream of the Ang1/Tie2 pathway may contribute to HGF gene induction.
Ang1 induced HGF expression in HUVECs. (A) Total RNAs were extracted from corresponding adenoviral vector–infected HUVECs for 48 hours. HGF mRNA was analyzed by Northern blot. 18S rRNA, visualized with ethidium bromide and UV, was used as a loading control. Images are representative of 3 separate experiments. (B) Cells were lysed from corresponding adenoviral vector–infected HUVECs for 48 hours. Cell lysates were analyzed by Western blotting and probed with an anti-HGF antibody. (C) Cell-conditioned media were collected, concentrated, and analyzed for protein expression by Western blotting. Filters were immunoblotted with anti-Ang1–, anti-Ang2–, and anti-VEGF–specific antibodies, respectively.
Ang1 induced HGF expression in HUVECs. (A) Total RNAs were extracted from corresponding adenoviral vector–infected HUVECs for 48 hours. HGF mRNA was analyzed by Northern blot. 18S rRNA, visualized with ethidium bromide and UV, was used as a loading control. Images are representative of 3 separate experiments. (B) Cells were lysed from corresponding adenoviral vector–infected HUVECs for 48 hours. Cell lysates were analyzed by Western blotting and probed with an anti-HGF antibody. (C) Cell-conditioned media were collected, concentrated, and analyzed for protein expression by Western blotting. Filters were immunoblotted with anti-Ang1–, anti-Ang2–, and anti-VEGF–specific antibodies, respectively.
Ang1 induced smooth muscle cell migration via HGF up-regulation
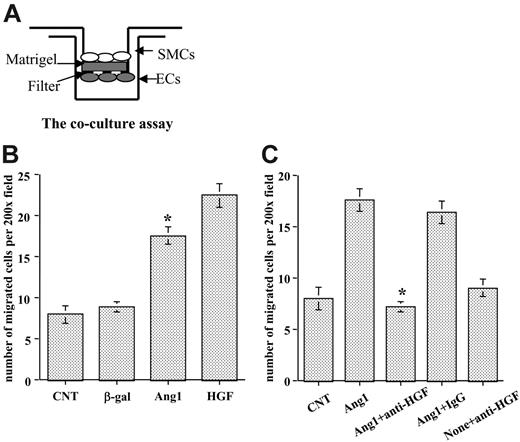
The role of HGF in regulating SMC motility has been relatively well established.14-16 After observing that Ang1 induced HGF expression in endothelial cells, we examined whether Ang1 regulated SMC recruitment through HGF production. We developed a coculture assay, based on the Transwell assay (Figure 3A), to test the hypothesis. HUVECs were infected with different viral vectors expressing the gene of interest and then were placed on the underside of Transwell filters to prevent the dilution of HGF in the lower wells. HASMCs were labeled with RFP by cell infection with an AdRFP viral vector and then were seeded on top of the filter in the upper chamber. For positive control, we added human recombinant HGF at 50 ng/mL in the bottom chamber. SMCs that migrated to the other side of the filter were counted after 5 hours of incubation. The data showed that significantly more HASMCs migrated when cocultured with Ang1-stimulated HUVECs than when cells were treated with the control vectors (P < .01). Control viral vector did not affect SMC migration compared with the noninfected control (Figure 3B).
To determine whether Ang1 induced SMC recruitment through the production of HGF, we included a specific neutralizing anti-HGF antibody in the experiment. Once again, Ang1-stimulated ECs significantly increased HASMC migration. Interestingly, neutralizing HGF function completely blocked HASMC migration caused by Ang1-stimulated endothelial cells compared with Ang1-treated cells (P < .01). Control IgG had no effect on SMC migration (Figure 3C). Thus, the data demonstrate that SMC migration induced by Ang1-stimulated ECs is mediated through HGF production.
Ang1 induced HGF production independently of Akt, MAPK, and p38 MAPK activation. (A) Total RNAs were extracted from corresponding adenoviral vector–infected HUVECs at 16, 24, and 48 hours, respectively. HGF mRNA was analyzed by Northern blot. 18S rRNA, visualized with ethidium bromide and UV, was used as a loading control. Images are representative of 3 separate experiments. (B) Inactivation of dominant-negative Akt was confirmed by Akt kinase assay using cells treated in the same manner as in panel A. (C) HUVECs were treated with PD98059 at 20 μM for 30 minutes before adenoviral-Ang1* infection for 16, 24, and 48 hours, respectively. Northern blot analysis was performed using total RNAs. Images are representative of 3 separate experiments. (D) Effectiveness of PD98059 was tested in the cells treated as in panel C. As a positive control, FGF was used. Extracted protein was analyzed by Western blot. (E) HUVECs were treated with SB203580 at 10 μM for 30 minutes before adenoviral-Ang1* infection for 16, 24, and 48 hours, and Northern blot was performed.
Ang1 induced HGF production independently of Akt, MAPK, and p38 MAPK activation. (A) Total RNAs were extracted from corresponding adenoviral vector–infected HUVECs at 16, 24, and 48 hours, respectively. HGF mRNA was analyzed by Northern blot. 18S rRNA, visualized with ethidium bromide and UV, was used as a loading control. Images are representative of 3 separate experiments. (B) Inactivation of dominant-negative Akt was confirmed by Akt kinase assay using cells treated in the same manner as in panel A. (C) HUVECs were treated with PD98059 at 20 μM for 30 minutes before adenoviral-Ang1* infection for 16, 24, and 48 hours, respectively. Northern blot analysis was performed using total RNAs. Images are representative of 3 separate experiments. (D) Effectiveness of PD98059 was tested in the cells treated as in panel C. As a positive control, FGF was used. Extracted protein was analyzed by Western blot. (E) HUVECs were treated with SB203580 at 10 μM for 30 minutes before adenoviral-Ang1* infection for 16, 24, and 48 hours, and Northern blot was performed.
Stimulation of endothelial cells with Ang1 induced SMC migration through HGF up-regulation. (A) Coculture assay using Transwell was developed to evaluate SMC migration. Both sides of the filters were coated with Matrigel. ECs were seeded underneath the filter, and SMCs were seeded in the upper chamber. (B) HUVECs were infected with various viral vectors expressing genes of interest for 48 hours, and SMCs were labeled with RFP. Then the coculture assay was set up as shown in panel A. Migrated SMCs on the other side of the filter were counted through a microscope in randomly selected high-power fields. (C) Effects of neutralization of HGF function in EC-SMC recruitment were evaluated with the coculture assay. Neutralizing HGF antibody or control IgG was added 1 hour before HASMCs were added to the wells. Experiments were performed at least 3 times in more than 2 wells for each treatment. Five fields were counted for each filter in each experiment. *P < .01 compared with control.
Stimulation of endothelial cells with Ang1 induced SMC migration through HGF up-regulation. (A) Coculture assay using Transwell was developed to evaluate SMC migration. Both sides of the filters were coated with Matrigel. ECs were seeded underneath the filter, and SMCs were seeded in the upper chamber. (B) HUVECs were infected with various viral vectors expressing genes of interest for 48 hours, and SMCs were labeled with RFP. Then the coculture assay was set up as shown in panel A. Migrated SMCs on the other side of the filter were counted through a microscope in randomly selected high-power fields. (C) Effects of neutralization of HGF function in EC-SMC recruitment were evaluated with the coculture assay. Neutralizing HGF antibody or control IgG was added 1 hour before HASMCs were added to the wells. Experiments were performed at least 3 times in more than 2 wells for each treatment. Five fields were counted for each filter in each experiment. *P < .01 compared with control.
Ang2 inhibited Ang1-induced HGF transcription
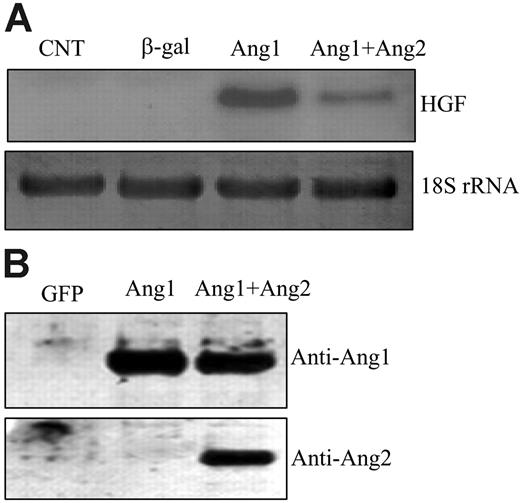
Genetic evidence and biochemical data suggest that Ang2 is an antagonist ligand that inhibits Ang1-mediated Tie2 activation.29 Ang2 is mainly expressed in growing vessels, whereas Ang1 is mainly expressed in mature vessels, indicating that Ang1 and Ang2 may have opposing effects in the regulation of SMC recruitment.5 Therefore, we studied the effects of Ang2 on HGF expression. We coinfected HUVECs with AdAng1 and AdAng2 virus and then examined HGF expression. As expected, stimulation of ECs with Ang1 induced HGF production. Coexpression of Ang2 significantly inhibited the Ang1-induced HGF transcription 3.0 ± 0.2-fold compared with Ang1 alone (n = 6; P < .01) (Figure 4A), which is in agreement with the notion that Ang2 is an antagonist ligand. Overexpression of Ang2 alone did not affect HGF expression in endothelial cells (Figure 1A). To ensure that HGF induction was not caused by viral infection, we treated the cells with the conditioned media collected from each group. Results identical to those of viral infection were observed: Ang1-conditioned media induced HGF expression, and Ang2-conditioned media blocked Ang1-induced HGF production in endothelial cells (data not shown). In addition, cell culture media from different viral vector–infected cells were collected, concentrated, and analyzed for Ang1 and Ang2 expression. The data confirmed that Ang1 and Ang2 proteins were correctly expressed in each group (Figure 4B).
Ang2 inhibited Ang1-induced HGF expression in endothelial cells. (A) HUVECs were infected with different viral vectors expressing genes of interest for 48 hours. Total RNAs were extracted from the cells. HGF mRNA was analyzed by Northern blotting and was probed with HGF cDNA. 18S rRNA, visualized with ethidium bromide and UV, was used as a loading control. (B) Cell-conditioned media were collected, concentrated, and analyzed for protein expression by Western blotting. Filters were immunoblotted with anti-Ang1– and anti-Ang2–specific antibodies, respectively. Representative images are shown. Experiments were performed at least 3 times.
Ang2 inhibited Ang1-induced HGF expression in endothelial cells. (A) HUVECs were infected with different viral vectors expressing genes of interest for 48 hours. Total RNAs were extracted from the cells. HGF mRNA was analyzed by Northern blotting and was probed with HGF cDNA. 18S rRNA, visualized with ethidium bromide and UV, was used as a loading control. (B) Cell-conditioned media were collected, concentrated, and analyzed for protein expression by Western blotting. Filters were immunoblotted with anti-Ang1– and anti-Ang2–specific antibodies, respectively. Representative images are shown. Experiments were performed at least 3 times.
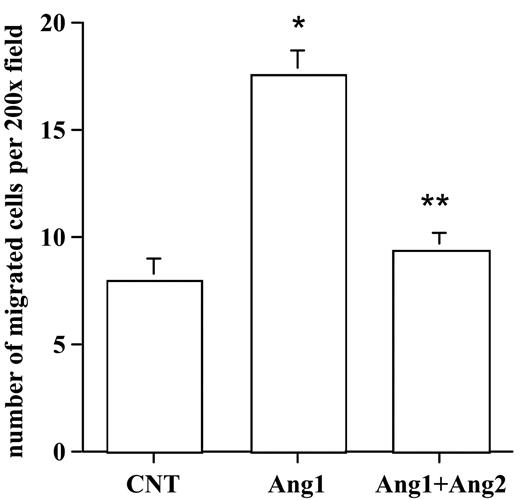
Ang2 neutralized Ang1-mediated SMC migration
Next, we examined the effects of Ang2 on Ang1-mediated SMC migration with the use of the coculture assay. HUVECs were infected with control, AdAng1, or AdAng1 plus AdAng2, followed by the establishment of the SMC-EC Transwell assay. The number of migrated HASMCs was determined after 5 hours of incubation. As expected, stimulation of endothelial cells with Ang1 significantly increased SMC migration compared with control (P < .01). Interestingly, coexpression of Ang2 suppressed HASMC recruitment induced by Ang1 significantly more than by Ang1 alone (P < .01) (Figure 5). Taken together, these findings clearly established a yin-yang regulation mechanism of Ang1 and Ang2 in HGF expression in endothelial cells that resulted in smooth muscle cell recruitment during vascular maturation.
Yolk sac endothelial cells express HGF and inactivation of Tie2 signaling in in vivo down-regulated HGF production
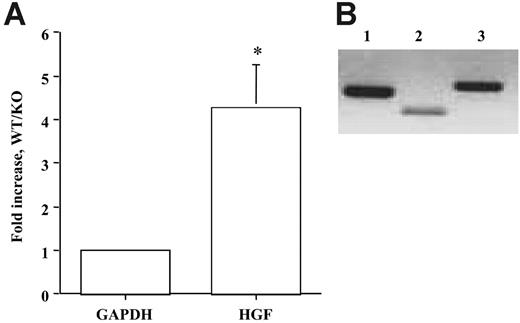
Genetic evidence shows that inactivation of Ang/Tie2 in transgenic mouse model resulted in vascular defects characterized by reduced SMC recruitment and vascular maturation in embryos,4,7,30,31 indicating a role for Ang/Tie2 signaling in vascular remodeling. Because our in vitro data indicated Ang/Tie2 signaling regulated SMC recruitment through HGF production, we reasoned that HGF production might contribute to reduced SMC recruitment in Tie2 null mice. To test the hypothesis, we analyzed HGF production in wild-type and Tie2 knockout embryo yolk sacs harvested at day 9.5 by real-time RT-PCR. We found more than 4-fold decreases of HGF expression in Tie2 null embryos compared with wild-type controls (Figure 6A; P < .05). This result supports the hypothesis that Tie2 signaling regulates SMC recruitment through HGF production in vivo.
Studies have clearly shown that Tie2 is specifically expressed in vascular ECs, Ang1 is expressed mainly in SMCs and pericytes, and Ang2 is expressed in ECs and SMCs. To determine the cellular expression of HGF, RT-PCR analysis was performed on yolk sac endothelial cells (C166).19 Two sets of HGF primers, corresponding to 300-bp and 500-bp fragments, were used to increase the specificity of the experiment. We found that yolk sac endothelial cells expressed HGF (Figure 6B). Collectively, these findings provided physiologic evidence supporting our hypothesis that HGF mediates Ang/Tie2 signaling-induced SMC recruitment and vascular maturation in vivo.
Ang2 antagonized Ang1 stimulation of endothelial cell–induced SMC migration. HUVECs were infected with a control vector, AdAng1, and AdAng1 plus AdAng2 for 48 hours. SMC migration was evaluated with the EC-SMC coculture assay. Migrated SMCs were counted in randomly selected fields. Experiments were repeated at least 3 times in more than 2 wells for each treatment. Five fields were counted for each filter in each experiment. *P < .01 compared with control. **P < .01 compared with Ang1 group.
Ang2 antagonized Ang1 stimulation of endothelial cell–induced SMC migration. HUVECs were infected with a control vector, AdAng1, and AdAng1 plus AdAng2 for 48 hours. SMC migration was evaluated with the EC-SMC coculture assay. Migrated SMCs were counted in randomly selected fields. Experiments were repeated at least 3 times in more than 2 wells for each treatment. Five fields were counted for each filter in each experiment. *P < .01 compared with control. **P < .01 compared with Ang1 group.
Quantification of HGF levels in Tie2 KO embryos. (A) Real time RT-PCR analysis on HGF levels was performed on wild-type and Tie2 knockout embryo yolk sacs harvested at day 9.5. Gene expression levels were normalized according to the expression of the housekeeping gene GAPDH. HGF expression was decreased 4.26-fold in Tie2-null yolk sacs compared with wild-type controls. Three pairs of embryo tissues were used in the study. RT-PCR was performed in triplicate and with 3 different dilutions of cDNA. *P < .01 compared with control. (B) RT-PCR analysis on HGF was also performed on endothelial cells isolated from the wild-type yolk sac (C166). Two sets of primers for HGF were used to increase specificity. HGF expression was confirmed (lane 2, 300 bp; lane 3, 500 bp). GAPDH was used as a control (lane 1, 440 bp).
Quantification of HGF levels in Tie2 KO embryos. (A) Real time RT-PCR analysis on HGF levels was performed on wild-type and Tie2 knockout embryo yolk sacs harvested at day 9.5. Gene expression levels were normalized according to the expression of the housekeeping gene GAPDH. HGF expression was decreased 4.26-fold in Tie2-null yolk sacs compared with wild-type controls. Three pairs of embryo tissues were used in the study. RT-PCR was performed in triplicate and with 3 different dilutions of cDNA. *P < .01 compared with control. (B) RT-PCR analysis on HGF was also performed on endothelial cells isolated from the wild-type yolk sac (C166). Two sets of primers for HGF were used to increase specificity. HGF expression was confirmed (lane 2, 300 bp; lane 3, 500 bp). GAPDH was used as a control (lane 1, 440 bp).
Discussion
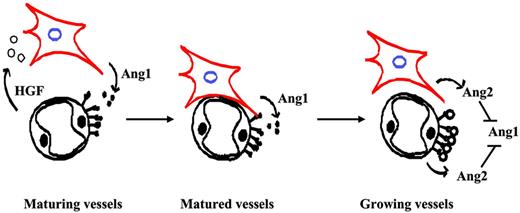
Blood vessel walls are composed of ECs and SMCs. During vascular maturation, ECs recruit SMCs to form stable and functional blood vessels. In contrast, during angiogenesis, these 2 cells dissociate from each other and create space for ECs to grow and form vascular tubules. Clearly, communication between these 2 types of cells is critical for regulating vascular maturation. Here, we studied the role of Ang/Tie2 signaling in vascular maturation. We identified a novel regulation mechanism of angiopoietins in regulating SMC recruitment. Our data showed that the stimulation of ECs with an agonist ligand of Tie2, Ang1, induced HGF production, which in turn led to SMC migration toward ECs. On the other hand, Ang2, an antagonist ligand of Tie2, neutralized Ang1-induced HGF expression in endothelial cells and SMC recruitment. Therefore, our data present a yin-yang regulation mechanism of angiopoietins in SMC recruitment (Figure 7).
Genetic studies have implicated that Ang/Tie2 signaling regulates SMC recruitment during vascular maturation.4-6 Inactivation of either Tie2 or Ang1 in transgenic mice leads to vascular abnormalities with reduction or lack of SMCs in the vasculature.4,6 Conversely, activating Tie2 caused venous malformation with multiple layers of SMCs in venous walls.7 Analysis of mice lacking Ang2 reveals that Ang2 is dispensable for embryonic vascular formation but is essential for subsequent vascular maturation.32 Genetic rescue with Ang1 failed to correct the angiogenic defects, suggesting that Ang2 acts as an antagonist during the process. The common theory is that Ang2 may collaborate with VEGF to promote angiogenic sprouting from an established vasculature.5,33-35 Reciprocally, Ang2 destabilizes the vasculature and thus promotes its regression in the absence of VEGF. Our findings provide molecular evidence to support this theory. Ang1 recruits SMCs through HGF production to stabilize the vessels during vascular maturation. Ang2 antagonizes the Ang1 function, inhibits Ang1-induced SMC recruitment, and destabilizes vessels. The destabilized endothelium could undergo either angiogenesis with the presence of VEGF or regression in the absence of VEGF. Our in vivo analysis, using Tie2 null mouse embryos, provides physiologic evidence to support that angiopoietins regulate SMC recruitment through HGF production. It would be interesting to test whether adding HGF could rescue the phenotypes of mouse embryos in the future.
Working model based on results with HUVECs and HASMCs. Pericytes/SMCs expressed Ang1, which up-regulated HGF expression in surrounding endothelial cells and resulted in pericyte/SMC recruitment toward endothelial cells. Ang2 antagonized Ang1-induced HGF expression and inhibited Ang1-induced SMC recruitment.
Working model based on results with HUVECs and HASMCs. Pericytes/SMCs expressed Ang1, which up-regulated HGF expression in surrounding endothelial cells and resulted in pericyte/SMC recruitment toward endothelial cells. Ang2 antagonized Ang1-induced HGF expression and inhibited Ang1-induced SMC recruitment.
PDGF-B has been shown to regulate endothelial cell-induced recruitment and differentiation of SMCs in cell culture systems.36,37 Inactivation of PDGF-B in transgenic mice displays vascular defects with loss of pericytes.38 In an earlier study, we attempted to examine the contribution of PDGF-B in Ang1-induced SMC recruitment.39 Ang1 stimulation caused limited down-regulation of PDGF-B expression in endothelial cells rather than up-regulation of PDGF-B, as we expected, which suggests it is less likely that PDGF-B contributes to the SMC recruitment in response to Ang1. To search for the candidate gene responsible for this function, we used microarray analysis and identified HGF, a potent factor that regulates SMC motility in different models,14-16 as a potential candidate. Our results indeed confirmed that Ang1 induced HGF expression in endothelial cells and consequently led to SMC recruitment. Ang2 blocked Ang1-induced HGF production and SMC recruitment.
HGF, a cytokine of mesenchymal origin, elicits a wide spectrum of biologic activities that include smooth muscle cell migration.14-16 The expression of 6.0-kb and 3.0-kb full-length HGF mRNA and alternatively spliced 1.5-kb mRNA has been reported in a human embryonic fibroblast cell line.40 However, we only detected a 3.0-kb mRNA in cultured endothelial cells, indicating that different types of cells may express different HGF isoforms. HGF is usually produced in mesenchymal cells. In this study, we showed that HGF is expressed in endothelial cells, including cells derived from yolk sac. We also found that HGF could be induced in endothelial cells upon stimulation with Ang1. A recent study showed that VEGF induces HGF in liver sinusoidal cells and protects the liver from CCl4 toxicity.41 However, we observed limited HGF gene induction in HUVECs and RMECs stimulated with VEGF.41 Heterogeneity of endothelial cells from different organs has been well documented, which could explain the discrepancy of different endothelial cells responding to different growth factors.42 VEGF induction of HGF might be limited to specific organs and thus might have therapeutic advantages in these organs.
PI3K/Akt, MAPK/ERK, and p38 MAPK are 3 major downstream signaling mediators in endothelial cells that could be activated upon stimulation with Ang1, which renders endothelial cells with a variety of biologic functions.24,25,43,44 Therefore, we examined the contribution of these 3 mediators in HGF production in cultured endothelial cells in response to Ang1 stimulation. However, the inhibition of Akt, ERK, or p38 MAPK with specific inhibitors did not have any effects on HGF induction in endothelial cells (Figure 2), suggesting that other factors could contribute this gene regulation. Current efforts are directed toward identifying signaling mediators responsible for Ang1-mediated HGF expression in endothelial cells.
In conclusion, we demonstrate for the first time that Ang1 induces HGF expression and that Ang2 inhibits HGF expression induced by Ang1 in cultured endothelial cells, corresponding to increased or decreased SMC recruitment. We also showed that yolk sac endothelial cells express HGF and that the inactivation of Tie2 in vivo significantly reduced HGF production, which could explain the deficient SMC recruitment in Tie2 null mice. These findings establish an intriguing and delicate regulation mechanism of angiopoietins in SMC recruitment during vascular formation and remodeling.
Prepublished online as Blood First Edition Paper, April 25, 2006; DOI 10.1182/blood-2005-09-012807.
Supported in part by the Vanderbilt-Ingram Cancer Center and the T. J. Martell Foundation and by National Cancer Institute grants CA108856 and NS45888 (P.C.L.) and training grants CA093240 and CA09592 (H.K., L.M.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Michael Freeman and Mark deCaestecker at Vanderbilt University Medical Center for their critical reading of and their comments on the manuscript. We thank the Vanderbilt Microarray Core for technical support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal