Comment on Tefferi et al, page 1158
Elevated serum VEGF and neoangiogenesis are characteristic features of idiopathic myelofibrosis (IMF), and in small phase 2 trials the antiangiogenic agent thalidomide demonstrated efficacy in correcting anemia and thrombocytopenia in IMF but with substantial toxicity.
Lenalidomide, a potent thalidomide analog devoid of neurotoxicity, produced significant cytogenetic and hematologic responses in myelodysplasia (MDS), including resolution of myelofibrosis.1 In this issue of Blood, Tefferi and colleagues describe 2 small lenalidomide phase 2 trials in chronic myeloproliferative disorder (MPD) patients with myelofibrosis and myeloid metaplasia (MMM).
The chronic MPD, idiopathic myelofibrosis (IMF), polycythemia vera (PV), and essential thrombocytosis (ET) share in common origin in a multipotent hematopoietic stem cell and overproduction of one or more blood cell types. The 3 are also united by JAK2V617F expression, impaired Mpl expression, in vitro autonomous hematopoietic colony formation, and a tendency to mimic each other phenotypically. PV and ET are disorders of substantial longevity for which there is established therapy. By contrast, other than stem cell transplantation, there is no effective treatment for IMF, which often has an inexorable course and reduced life expectancy; PV with MMM represents a similar problem.
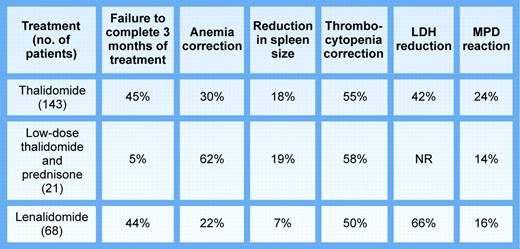
Compared with thalidomide (see figure), lenalidomide was not more efficacious in IMF or MMM-complicating PV, with the expected exception of one patient with del(5)(q13q33). However, firm conclusions about lack of efficacy from such phase 2 data would be misleading. First, patient numbers were small and the populations studied heterogenous, since the data suggest that IMF and MMM-complicating PV are not similar entities, while ET with MMM is either IMF presenting with thrombocytosis2 or preleukemic transformation. Second, the trials were neither blinded nor randomized, rendering assessment of transfusion requirements, spleen size, and marrow angiogenesis suspect. Third, these patients, as in other studies of this type, had been previously heavily treated, including with thalidomide. Indeed, only patients without prior thalidomide exposure responded to lenalidomide. Fourth, the duration of therapy may have been too short. Fifth, disease duration obviously varied and the disease stage, either in IMF or PV, might have been too advanced for lenalidomide or any biologic response modifier to be effective. This is not to fault the authors for conducting these trials. Given lack of effective therapy for IMF or MMM-complicating PV and lenalidomide's demonstrated effectiveness in MDS, any of us would have welcomed the opportunity to use this drug.FIG1
Published phase 2 trials of thalidomide and lenalidomide in myeloid metaplasia. Illustration by Kenneth Probst.
Published phase 2 trials of thalidomide and lenalidomide in myeloid metaplasia. Illustration by Kenneth Probst.
The overriding issues here are actually different. First, IMF, PV, and ET are sufficiently uncommon disorders that if we are to learn anything about them, their treatment should be considered experimental and patients from the time of diagnosis should be offered enrollment in a clinical trials network similar to those pioneered by our overseas colleagues.3,4 The recent initiation of a National Institutes of Health (NIH)–sponsored MPD research consortium should facilitate this. Second, we need to move away from the thinking of 50 years ago; myelofibrosis is not the culprit. Myelofibrosis is a reactive, reversible phenomenon. Myeloid metaplasia is the real problem, reflecting a profound disturbance in stem cell physiology that we do not yet understand. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal