Abstract
Matk/CHK knockout mice were reported to show no apparent phenotypic abnormalities. This was thought to be due to the homologous kinase Csk that compensates for Matk/CHK. Here, we present the first evidence that the nonreceptor tyrosine kinase, Matk/CHK, is an important modulator of immune cell signaling. We found that the frequency of primitive hematopoietic cells, the side population c-kit+ Lin– Sca-1+ (SPKLS) cells, in Matk/CHK–/– mice was increased 2.2-fold compared with the control mice. Moreover, Matk/CHK deficiency led to significantly higher pre–B cell colony formation following IL-7 stimulation. Interestingly, when mice received the in vivo antigen challenge of TNP-ovalbumin followed by restimulation, the Matk/CHK–/– lymph node and spleen cells produced significantly lower IFN-γ levels compared with the respective wild-type cells. Our study indicates that Matk/CHK is not functionally redundant with Csk, and that this tyrosine kinase plays an important role as a regulator of immunologic responses.
Introduction
Tyrosine kinases are essential regulators of immune responses and play important roles in directing hematopoietic progenitors into functional hematopoietic cells. Abnormalities of tyrosine phosphorylation in hematopoietic cells lead to hyperproliferative disorders and to abnormal development of hematopoietic cells, such as in leukemia and autoimmune disease.1,2
Megakaryocyte-associated tyrosine kinase (MATK), also termed Csk homologous kinase (CHK), is a nonreceptor tyrosine kinase that negatively regulates the activity of Src family protein tyrosine kinases.3 MATK/CHK shares approximately 50% homology with Csk.3 Like Csk, MATK/CHK has Src homology 3 (SH3) and SH2 domains and lacks the consensus tyrosine phosphorylation and myristylation sites found in Src family kinases.4 However, in contrast to their structural similarities, the expression patterns of the 2 kinases are quite different. Whereas Csk is ubiquitously expressed, MATK/CHK is expressed mainly in hematopoietic cells and brain.5 Additionally, whereas Csk homozygous mutant embryos exhibit a complex phenotype (including defects in the neural tube) and die between days 9 and 10 of gestation,6 Matk/CHK knockout mice are fully immunocompetent, have normal peripheral hematopoietic cell populations, a normal life span, and are fertile.5,7
Despite the restricted expression of MATK/CHK in brain and hematopoietic cells, its role in these cells has not been studied extensively. This is due partly to the absence of an overt phenotype in the Matk/CHK knockout (KO, –/–) mice.5,7 Here, we redefined the role of MATK/CHK using Matk/CHK–/– mice. Whereas Matk/CHK–/– mice appear to be normal in the steady state, stimulation of the hematopoietic cells of these mice with IL-7 and in vivo challenge with antigen (TNP-ovalbumin [TNP-Ova]) led to strikingly different physiologic responses, compared with the similarly treated hematopoietic cells of the Matk/CHK+/+ mice. Our results provide new insights into the molecular mechanisms through which MATK/CHK is involved in immune responses.
Study design
Mice
A Matk null mutation (Matktm1Sor) was generated in the embryonic stem (ES) cell line AK7 (129S4/SvJaeSor background) using homologous recombination to replace with a neo cassette an approximately 500-bp fragment including exon 7 that encodes the amino acid sequence essential for ATP binding. Heterozygous mice were bred by continual backcross with C57BL/6J mice for more than 11 generations. Homozygous mice were obtained by crossing heterozygous mice, and then maintained by brother-sister mating between the homozygotes. The genotypes of these mice were determined by polymerase chain reaction (PCR) using 3 primers: Ctk e6F (5′-GAT TGG AGA GGG GGA GTT-3′), Ctk i7R (5′-TGG GCA GAG AGT TGG AAA-3′), and no. 9603 (5′-TCA TAG CCG AAT AGC CTC TCC AC-3′).
Colony assay
Multilineage progenitor (CFU-Mix) and pre-B colony assays were performed as described.8
Immunization and cytokine measurement
A total of 200 μg TNP-Ova (Biosearch Technologies, Novato, CA) emulsified in incomplete Freund adjuvant was injected subcutaneously into the back and in both hind footpads of Matk/CHK+/+ and Matk/CHK–/– mice. At 7 to 10 days after immunization, the lymph node or spleen cells was plated at 2 × 106 cells/mL in RPMI-1640 with 10% FBS. Variable concentrations of ovalbumin were added to each well. Supernatants from each well were collected every 24 hours for 3 days and cytokine concentrations were measured using an enzyme-linked immunosorbent assay (ELISA) kit (eBioScience, San Diego, CA).
Statistics
Statistical analysis was performed using the Student t test. P values below .05 were considered significant.
Results and discussion
Protein tyrosine kinases are known to play a key role in mediating immune signaling and disease progression.9 Studies of knockout mice lacking various tyrosine kinases have confirmed their roles as important modulators of immune cell signaling.10-12 MATK/CHK is a tyrosine kinase that is expressed in hematopoietic cells.13 Mice lacking the expression of Matk/CHK have been demonstrated to have no overt phenotype.5,7 Those earlier studies focused on hematopoietic cells due to the restricted expression of Matk/CHK in these cells. Since then, more lineage markers have been defined, which allow us to supplement the prior hematopoietic cell analyses. We analyzed the phenotype of hematopoietic cells in bone marrow (BM), spleen, and thymus, using lineage-specific antibodies. CD19 and B220 (CD45R) are surface markers of B cells, and B220 expression precedes CD19 expression during early B-cell development.14 When mononuclear cells from Matk/CHK–/– mice were analyzed with CD19 and B220, there was no significant difference observed between the Matk/CHK–/– and Matk/CHK+/+ (WT) mice. T cells develop through 3 stages defined by the presence of 2 glycoproteins, CD4 and CD8. Fluorescence activated cell sorting (FACS) analysis using anti-CD4 and anti-CD8 revealed no difference between the double-negative (DN), double-positive (DP), and single-positive T cells from the Matk/CHK–/– and WT mice. Moreover, FACS analysis of monocytic (CD11b+/GR1–) and granulocytic (CD11b/GR1 double-positive) cells from Matk/CHK–/– mice revealed no abnormal patterns compared with the analysis of the WT mice. In summary, the pattern of lineage marker expression in the Matk/CHK–/– mice was indistinguishable from that of the WT mice.
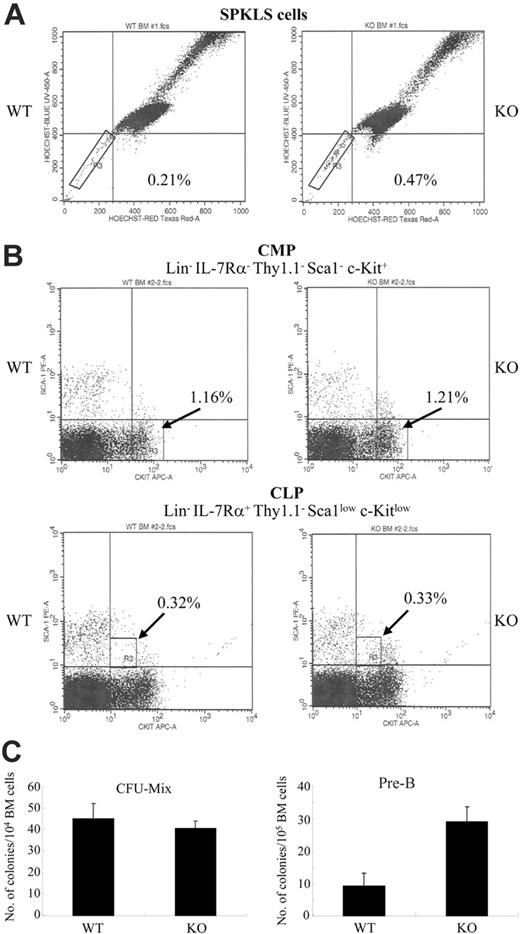
However, using recently defined stem cell markers to study primitive hematopoietic stem cell populations (SPKLS), we found that Matk/CHK–/– mice have an approximately 2-fold higher SPKLS cell population (c-Kit+, Lin–, Sca-1+ in combination with side population cells)15 than WT mice (Figure 1A). Further study of the primitive stem cell population in Matk/CHK–/– bone marrow, using extended long-term culture-initiating cells (LTCICs) and in vivo repopulation assays, will assist in determining the functional role of MATK/CHK in hematopoietic stem cells.
Analysis of common myeloid progenitors (CMPs) and common lymphoid progenitors (CLPs) in the BM of Matk/CHK–/– and WT mice showed no differences (Figure 1B). Additionally, no differences were observed in the number of CFU-Mix colonies between the Matk/CHK–/– and WT mice (Figure 1C). However, Matk/CHK knockout BM cells showed greatly enhanced proliferative expansion in vitro and a higher number of pre-B colonies in the presence of IL-7, compared with WT BM cells (Figure 1C). This result was not due to the different frequency of cells expressing the IL-7 receptor or to the elevated levels of B-cell progenitors in the Matk/CHK–/– BM cells (data not shown). Therefore, although MATK/CHK deficiency did not perturb the normal pattern of the major lymphocytes, our results showed an as-yet-unknown function of MATK/CHK as a negative regulator in pre–B cell proliferation.
Deficiency in the Matk/CHK gene alters early hematopoietic stem cell populations and leads to the hyperproliferation of pre-B cells in the presence of IL-7. (A) Frequency of SPKLS cells in the Matk/CHK–/– mice. BM cells from Matk/CHK+/+ (WT) and Matk/CHK–/– (KO) mice were stained as described.15 Lineage-positive cells were depleted using magnetic columns and a cocktail of biotinylated lineage-specific antibodies. The BM cells were stained with Hoechst 33342 followed by antibody staining (anti–Sca-1 and anti–c-Kit). The boxed regions define the SPKLS cells. The results shown are representative of 3 independent experiments. The lack of Matk/CHK protein in the homozygous mice was confirmed by Western blot analysis of adult brain samples (not shown), which normally express high levels of Matk/CHK. (B) Frequency of CMP and CLP in the Matk/CHK–/– mice. BM cells from the Matk/CHK+/+ (WT) and Matk/CHK–/– (KO) mice were stained as described.11 Lineage-positive cells were depleted using magnetic columns and a cocktail of biotinylated lineage-specific antibodies. CMP was defined by the Lin– IL-7Rα–Thy-1–Sca1–c-Kit+ phenotype. CLP was defined by the Lin– IL-7Rα+ Thy-1– Sca1low c-Kitlow phenotype. The results shown are representative of 2 independent experiments. (C) Matk/CHK–/– mice produce a higher number of pre-B colonies, but not of the CFU-Mix. Total BM cells from Matk/CHK+/+ (WT) and Matk/CHK–/– (KO) mice were placed in methylcellulose cultures with a cocktail of cytokines either for the CFU-Mix or pre–B cell colony formation. The cells were plated in triplicate cultures. After 10 to 12 days of culture, pre–B cell colonies were counted. CFU-Mix colonies were counted after 10 to 14 days of culture. Analysis of colony formation was conducted using an inverted microscope. A colony was defined as consisting of at least 200 cells for the CFU-Mix and 50 cells for the pre-B colony. There were no significant differences between genotypes in the CFU-Mix assay (mean 27.4 versus 29.8, WT versus Matk/CHK–/– cells, based on 4 independent experiments). The number of pre-B colonies from the Matk/CHK-deficient BM cells was approximately 4-fold higher than that from the WT BM cells (mean 7.5 versus 29.7, WT versus Matk/CHK–/–, based on 3 independent experiments).
Deficiency in the Matk/CHK gene alters early hematopoietic stem cell populations and leads to the hyperproliferation of pre-B cells in the presence of IL-7. (A) Frequency of SPKLS cells in the Matk/CHK–/– mice. BM cells from Matk/CHK+/+ (WT) and Matk/CHK–/– (KO) mice were stained as described.15 Lineage-positive cells were depleted using magnetic columns and a cocktail of biotinylated lineage-specific antibodies. The BM cells were stained with Hoechst 33342 followed by antibody staining (anti–Sca-1 and anti–c-Kit). The boxed regions define the SPKLS cells. The results shown are representative of 3 independent experiments. The lack of Matk/CHK protein in the homozygous mice was confirmed by Western blot analysis of adult brain samples (not shown), which normally express high levels of Matk/CHK. (B) Frequency of CMP and CLP in the Matk/CHK–/– mice. BM cells from the Matk/CHK+/+ (WT) and Matk/CHK–/– (KO) mice were stained as described.11 Lineage-positive cells were depleted using magnetic columns and a cocktail of biotinylated lineage-specific antibodies. CMP was defined by the Lin– IL-7Rα–Thy-1–Sca1–c-Kit+ phenotype. CLP was defined by the Lin– IL-7Rα+ Thy-1– Sca1low c-Kitlow phenotype. The results shown are representative of 2 independent experiments. (C) Matk/CHK–/– mice produce a higher number of pre-B colonies, but not of the CFU-Mix. Total BM cells from Matk/CHK+/+ (WT) and Matk/CHK–/– (KO) mice were placed in methylcellulose cultures with a cocktail of cytokines either for the CFU-Mix or pre–B cell colony formation. The cells were plated in triplicate cultures. After 10 to 12 days of culture, pre–B cell colonies were counted. CFU-Mix colonies were counted after 10 to 14 days of culture. Analysis of colony formation was conducted using an inverted microscope. A colony was defined as consisting of at least 200 cells for the CFU-Mix and 50 cells for the pre-B colony. There were no significant differences between genotypes in the CFU-Mix assay (mean 27.4 versus 29.8, WT versus Matk/CHK–/– cells, based on 4 independent experiments). The number of pre-B colonies from the Matk/CHK-deficient BM cells was approximately 4-fold higher than that from the WT BM cells (mean 7.5 versus 29.7, WT versus Matk/CHK–/–, based on 3 independent experiments).
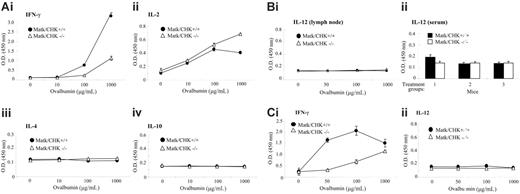
Deficiency in the Matk/CHK gene impairs the production of IFN-γ upon in vivo antigen challenge. (A) One week after immunization with TNP-Ova, lymph node cells were prepared from Matk/CHK+/+ and Matk/CHK–/– mice, and then challenged with varying concentrations of ovalbumin as indicated. Concentrations of the indicated cytokines (i-iv) were determined by ELISA assay in duplicate, as described in “Study design.” The means were derived from 3 animals in each group. The data (OD450 value at 100 μg/mL and 1000 μg/mL ovalbumin) are summarized in Table 1. Results are from at least 3 independent experiments. (B) IL-12 expression levels in the lymph nodes (i) and serum (ii) derived from Matk/CHK–/– mice and control mice, as determined by ELISA assay. The means were derived from 3 animals in each group. Results are from 3 independent experiments. (C) One week after immunization with TNP-Ova, spleen cells were prepared from Matk/CHK+/+ and Matk/CHK–/– mice, and then challenged with varying concentrations of ovalbumin as indicated. Concentrations of the indicated cytokines, IFN-γ (i) and IL-12 (ii), were determined by ELISA assay in triplicate as described in “Study design.” The means were derived from 3 animals in each group.
Deficiency in the Matk/CHK gene impairs the production of IFN-γ upon in vivo antigen challenge. (A) One week after immunization with TNP-Ova, lymph node cells were prepared from Matk/CHK+/+ and Matk/CHK–/– mice, and then challenged with varying concentrations of ovalbumin as indicated. Concentrations of the indicated cytokines (i-iv) were determined by ELISA assay in duplicate, as described in “Study design.” The means were derived from 3 animals in each group. The data (OD450 value at 100 μg/mL and 1000 μg/mL ovalbumin) are summarized in Table 1. Results are from at least 3 independent experiments. (B) IL-12 expression levels in the lymph nodes (i) and serum (ii) derived from Matk/CHK–/– mice and control mice, as determined by ELISA assay. The means were derived from 3 animals in each group. Results are from 3 independent experiments. (C) One week after immunization with TNP-Ova, spleen cells were prepared from Matk/CHK+/+ and Matk/CHK–/– mice, and then challenged with varying concentrations of ovalbumin as indicated. Concentrations of the indicated cytokines, IFN-γ (i) and IL-12 (ii), were determined by ELISA assay in triplicate as described in “Study design.” The means were derived from 3 animals in each group.
IL-4 was reported to induce the expression of MATK/CHK, and IFN-γ was found to suppress its expression,16 suggesting the involvement of MATK/CHK in Th cell polarization. We differentiated naive WT or Matk/CHK–/– CD4 T cells into Th1 and Th2 cells in vitro. Supernatants were then analyzed for Th1 (IL-2, IFN-γ) and Th2 (IL-4, IL-10) cytokines using ELISA assays. Neither Th1 nor Th2 cytokine secretion was affected in the Matk/CHK–/– mice compared with the WT mice. Next, we investigated the effects of MATK/CHK in vivo. We immunized mice with TNP-Ova in incomplete Freund adjuvant. One week later, cell proliferation and cytokine production were determined after TNP-Ova restimulation ex vivo. We did not detect any differential proliferation between the Matk/CHK–/– and WT mice. We observed that the Matk/CHK–/– mice secreted low levels of IL-10 and IL-4 without any significant difference in their concentrations (Figure 2Aiii-iv and Table 1), suggesting that the Th2 component in the Matk/CHK–/– mice was intact. Antigen activation stimulates the secretion of IL-2 as well as the expression of the IL-2 receptor in T cells. Cells from the Matk/CHK–/– mice secreted slightly higher amounts of IL-2 at 1000 μg/mL TNP-Ova (Figure 2Aii) upon antigen stimulation. Even though there is a significant positive correlation between IL-2 and IFN-γ production,17,18 the Matk/CHK–/– mice exhibited significantly decreased IFN-γ secretion compared with the WT mice (Figure 2Ai), suggesting that the impaired IFN-γ production in the Matk/CHK–/– mice is independent from IL-2.
Deficiency in the Matk/CHK gene impairs the production of IFN-γ upon in vivo antigen challenge
Ovalbumin dose and mice . | IFN-γ . | IL-2 . | IL-4 . | IL-10 . |
|---|---|---|---|---|
| 100 μg/mL | ||||
| CHK+/+ | 0.79 ± 0.03 | 0.44 ± 0.03 | 0.12 ± 0.01 | 0.16 ± 0.02 |
| CHK-/- | 0.24 ± 0.01 | 0.53 ± 0.02 | 0.12 ± 0.01 | 0.15 ± 0.01 |
| 1000 μg/mL | ||||
| CHK+/+ | 3.27 ± 0.07 | 0.40 ± 0.01 | 0.13 ± 0.01 | 0.16 ± 0.01 |
| CHK-/- | 1.14 ± 0.11 | 0.68 ± 0.01 | 0.12 ± 0.01 | 0.15 ± 0.01 |
Ovalbumin dose and mice . | IFN-γ . | IL-2 . | IL-4 . | IL-10 . |
|---|---|---|---|---|
| 100 μg/mL | ||||
| CHK+/+ | 0.79 ± 0.03 | 0.44 ± 0.03 | 0.12 ± 0.01 | 0.16 ± 0.02 |
| CHK-/- | 0.24 ± 0.01 | 0.53 ± 0.02 | 0.12 ± 0.01 | 0.15 ± 0.01 |
| 1000 μg/mL | ||||
| CHK+/+ | 3.27 ± 0.07 | 0.40 ± 0.01 | 0.13 ± 0.01 | 0.16 ± 0.01 |
| CHK-/- | 1.14 ± 0.11 | 0.68 ± 0.01 | 0.12 ± 0.01 | 0.15 ± 0.01 |
Numbers denote OD values at 450 nm.
Since IL-12 feeds back to up-regulate IFN-γ, IRF-1, and IL-12R,19 we measured IL-12 expression in the lymph nodes and serum of Matk/CHK–/– and WT mice. No differences were observed in IL-12 levels between these knockout and WT mice (Figure 2Bi,ii).
A similar pattern of decreased IFN-γ secretion was observed in antigen-challenged spleen cells from Matk/CHK–/– mice (Figure 2Ci vs Figure 2Ai). However, no differences in IL-12 secretion were noted between the Matk/CHK–/– and WT mice (Figure 2Cii). Thus, MATK/CHK's effects on IFN-γ secretion are independent from IL-12.
IFN-γ is predominantly a negative regulator of B-cell differentiation and proliferation,20 which are mediated by apoptotic cell death.21 Whether hyperproliferation of Matk/CHK–/– BM cells is due to impaired production of IFN-γ in the Matk/CHK–/– mice followed by less apoptotic cell death needs further investigation.
The absence of a phenotype in the Matk/CHK knockout mice led to the notion that Matk/CHK function can be compensated for by its homologous kinase molecule, Csk. Although a more detailed mechanism by which MATK/CHK affects IFN-γ secretion and the hyperproliferation of B-lymphoid cells remains to be established, our work provides the first evidence that MATK/CHK serves as a regulator of immune responses and is not functionally redundant with Csk. Our studies in conjunction with those by other investigators illustrate the complex regulatory pathway of MATK/CHK kinase in immune cells. 16,22
Prepublished online as Blood First Edition Paper, March 30, 2006; DOI 10.1182/blood-2005-12-4885.
Supported by National Institutes of Health grants HL080 699 (S.A.) and CA 096 805 (H.K.A.).
H.K.A. designed research, analyzed data, and wrote the paper; B.C.L. performed research and analyzed data; S.A. analyzed data and contributed new reagents or analytical tools; and A.I. contributed new reagents or analytical tools.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Wei Fu for his typing assistance; Janet Delahanty for editing the paper; Dr Chris Daly and David Dombkowski for technical help; and Philippe Soriano, Robert C. Penhallow, and Joseph B. Bolen for generating the mouse strain Matktm1Sor originally performed by Akira Imamoto in P. Soriano's laboratory.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal