Abstract
Dosing of enzyme replacement therapy (ERT) for Gaucher disease type 1 is still a subject of debate and varies from 15 to 130 U/kg/mo, making a huge economic difference of US $70 000 to US $380 000 (€55 000-300 000) per patient per year. To investigate whether this difference in dosing ultimately translates into a different response, we retrospectively compared long-term outcome of ERT at 2 large European treatment centers, Academic Medical Center, Amsterdam, The Netherlands (n = 49, median dose, 15-30 U/kg/4 wks) and Heinrich-Heine University, Duesseldorf, Germany (n = 57, median dose, 80 U/kg/4 wks). These adult cohorts had a similar genetic background. All follow-up parameters were matched separately at baseline, to avoid bias with respect to disease severity. Improvement in hemoglobin, platelet count, and hepatosplenomegaly was not significantly different between both cohorts, whereas plasma chitotriosidase and bone marrow involvement by magnetic resonance imaging improved more quickly and was more pronounced in the higher-dosed group. Major bone complications rarely occurred in both groups. In conclusion, different dosing regimens of ERT do not affect outcome of hematologic and visceral parameters, but higher dosing leads to accelerated decrease of chitotriosidase and better objective bone response in adult type 1 Gaucher disease.
Introduction
Gaucher disease type 1 is the most common lysosomal storage disorder. Deficiency of the lysosomal enzyme glucocerebrosidase leads to the accumulation of glucocerebroside in spleen, liver, and bone marrow.1,2 In the early 1990s, Gaucher disease was the first of the lysosomal storage disorders that could be treated successfully with enzyme replacement therapy (ERT), using mannose-terminated enzyme from placental tissue (alglucerase) or recombinant enzyme (imiglucerase, both manufactured by Genzyme, Cambridge, MA). Most patients show a remarkable clinical response to treatment, with normalization of blood counts, a reduction in liver and spleen size, and improvement in bone symptoms.3-11 Even after more than 13 years of experience, the most effective dosing regimen of ERT is still a subject of debate. Advocates of low-dosing regimens call attention to the extremely high costs of the enzyme, US $70 000 to US $380 000 (€55 000-300 000) per patient per year depending on dose and weight, in the absence of convincing evidence for superiority of high dosages.12 Others argue that high dosages are required for optimal effect in severe disease, especially bone disease or in children.13-15 Guidelines for dosing protocols are based on consensus rather than on scientific evidence.16-18 Prospective studies that compare different dosing regimens are limited. Altarescu et al13 found inferior responses using 10 U/kg per 2 weeks compared to 60 U/kg per 2 weeks, but the 2 groups were not matched for baseline disease severity, and biochemical and bone marrow assessments were not included. Beutler19 showed in a meta-analysis that no relationship between dose and reduction in liver size could be established. He argued that fractionation may be important when small doses are given,20 although Zimran et al21 did not find a significant difference in response between a once-fortnightly and a 3-times-weekly schedule of 30 U/kg/4 wks of cerezyme.
Currently, large differences exist in dosing regimens between treatment centers, even in the same area. An example is found in Western Europe, where patients at the Academic Medical Center (AMC) in The Netherlands are treated with an initial low dose (15-50 U/kg/4 wks, divided in 1- to 3-times-weekly doses), while patients treated at the Heinrich Heine University (HHU) in Germany, less then 225 km away, use initial dosages between 60 and 120 U/kg/4 wks. The aim of the study was to compare long-term outcome on hematological, visceral, biochemical, and skeletal parameters in relation to these different dosing schedules.
Patients, materials, and methods
Setting and patients
We conducted a retrospective comparative cohort study at 2 centers. A total of 106 adult Gaucher type 1 patients who started treatment between 1991 and 2002 in the referral centers for Gaucher disease in AMC, Amsterdam, The Netherlands, and in HHU, Duesseldorf, Germany, and who received ERT with an initial dose of no more than 50 U/kg/4 wks (AMC) or at least 60 U/kg/4 wks (HHU) were included in the study. A diagnosis of Gaucher disease was confirmed by measurement of deficient glucocerebrosidase activity in leukocytes22 and by genotyping.
Patients at AMC are treated according to an individualized low-dose protocol,5 which consists of an initial dose of 15 to 50 U/kg/4 wks, given initially at a 3-times-a-week (36 of 49 patients) or once-a-week schedule (13 of 49 patients), which is individually adjusted based on the response of hematologic and visceral parameters, chitotriosidase, and clinical bone disease. The patients who started on a 3-times-a-week schedule switched to a once-a-week schedule after 2 to 4 years of ERT for reasons of convenience. Patients treated in HHU start with a dose of 60 to 120 U/kg/4 wks, given every other week, with the higher initial doses in more severe disease, such as extensive organomegaly or bone disease. The dose is slowly decreased in some patients, who reach stable disease.
Data analysis and definition of therapeutic goals
Baseline data on sex, age, splenectomy, severity score index (SSI, as described by Zimran et al23 ), use of bisphosphonates for more than 2 years, dosing, and genotype were recorded. Follow-up parameters included hemoglobin, platelet count, plasma chitotriosidase levels, liver and spleen dimensions, clinical records on bone disease, and scoring of bone marrow involvement by magnetic resonance imaging (MRI) of the femora. To account for differences in extent of disease between both cohorts, baseline characteristics were analyzed by Mann-Whitney U test or by chi-square test. Since the AMC cohort included patients with more extensive disease, all parameters were analyzed separately and matched at baseline. Each parameter, except for bone disease, was analyzed in 2 separate ways. First, baseline values (range,12 months before up to 2 months after start of ERT for organ volumes and bone marrow burden (BMB); baseline or within 12 months before start of ERT for hemoglobin, platelet count, and chitotriosidase) and values after one year (range, 8-16 months after start of ERT) were determined. Differences between both cohorts after one year were assessed by Mann-Whitney U test. Second, for each parameter, therapeutic goals were defined and analyzed by life table analysis (Kaplan Meier). The results were expressed as share of patients reaching the therapeutic goal versus duration of ERT, reflected as median. Differences between the cohorts were determined by the log-rank test. Approval was obtained from the AMC and HHU Institutional Review Boards for this study. Informed consent was provided in accordance with the Declaration of Helsinki.
Hemoglobin and platelet count. Matched pairs were made according to baseline values and spleen status, since hematologic response is much faster in splenectomized patients with cytopenia as compared to nonsplenectomized patients.5,9 Patients with a baseline hemoglobin level of less than 12.0 g/dL (= 7.5 mM) were selected, and matched pairs were made according to baseline values (maximum difference of 0.5 g/dL) and spleen status. Similar matching was performed for patients with a platelet count of less than 100 × 109/L at baseline (maximum difference of 10 × 109/L). The time to reach a hemoglobin level of more than 12.0 g/dL or to reach a platelet count of more than 100 × 109/L was determined.
Liver and spleen size. Liver and spleen volumes at AMC were measured by spiral computed axial tomography, with a reported accuracy of 3% to 5%.24-26 At HHU, using ultrasound, the maximum midclavicular cranio-caudal (CC) diameter and antero-posterior (AP) diameter of the liver were determined. For determination of spleen size, the diameter at the hilus (width), as seen on the horizontal view, and the distance between both poles (length), as seen on the frontal view, were determined. To allow comparison of the visceral data created by 2 different methods, the above-described liver and spleen dimensions were determined on computed tomographic (CT) images in a group of 55 AMC patients (a total of 103 spiral CTs) and compared to organ volumes, as determined by CT. The best correlation was found for both liver and spleen index, which is calculated by multiplying the respective diameters, yielding a correlation of 0.862 and 0.958, respectively (P < .001). Formulas were deducted to calculate liver and spleen volume from the respective indices (M.d.F., unpublished results). Baseline values between the 2 groups did differ significantly (Table 1), since the AMC cohort included 20 patients with liver volumes above 3000 mL (11 splenectomized), whereas these were not found in the HHU cohort. Therefore, patients with a liver volume above 1750 mL or spleen volume above 250 mL were selected, and matched pairs were made according to baseline organ volumes (maximum baseline difference of 200 mL for liver volume and 300 mL for spleen volume) and spleen status. Baseline values versus values after 12 months of ERT were compared. The time to reach a 20% decrease from baseline in liver volume or 40% decrease in spleen volume was determined by Kaplan Meier analysis in patients with a baseline liver volume in the range of 1750 mL to 3000 mL or spleen volume in the range of 400 mL to 2300 mL. No correction for change in body weight was performed since in this adult population, body weight did not change significantly.
Patient characteristics of 106 adult Gaucher type 1 patients
. | AMC . | HHU . | P . |
|---|---|---|---|
| No. of patients | 49 | 57 | NA |
| Age in 2003, y, median (range) | 47.0 (21-74) | 49.0 (21-82) | NS |
| Men, no. (%) | 27 (55%) | 25 (43%) | NS |
| Splenectomies, no. (%) | 19 (39%) | 19 (33%) | NS |
| SSI, median (range) | 8 (3-18) | 7.0 (2-16) | NS |
| Hb, g/dL, median (range) | 12.0 (8.0-15.8) | 12.6 (7.7-16.5) | .028 |
| Platelet count × 109/L, median (range) | 93 (17-726) | 73 (9-463) | NS |
| Liver volume, mL, median (range) | 3095 (1504-6542) | 1741 (1048-2821) | < .001 |
| Spleen volume, mL, median (range) | 1400 (470-4821) | 1597 (365-4195) | NS |
| BMB score, median (range) | 6.5 (0-8) | 7 (3-8) | NS |
| Chitotriosidase, nmol/mL/h, median (range) | 16 703 (5316-102 324) | 11 869 (592-39 791) | .056 |
| Patients with a serious bone complication in the 10 y prior to start of ERT, no. (%) | 21 (43) | 25 (44) | NS |
| Start dose, U/kg/4 wk, median (range) | 15 (15-50) | 80 (60-120) | < .001 |
| ERT cost per y for a 70-kg (154-lb) patient at start dose, median, US $ (€) | 69.85 (55.165) | 376.44 (294.221) | NA |
. | AMC . | HHU . | P . |
|---|---|---|---|
| No. of patients | 49 | 57 | NA |
| Age in 2003, y, median (range) | 47.0 (21-74) | 49.0 (21-82) | NS |
| Men, no. (%) | 27 (55%) | 25 (43%) | NS |
| Splenectomies, no. (%) | 19 (39%) | 19 (33%) | NS |
| SSI, median (range) | 8 (3-18) | 7.0 (2-16) | NS |
| Hb, g/dL, median (range) | 12.0 (8.0-15.8) | 12.6 (7.7-16.5) | .028 |
| Platelet count × 109/L, median (range) | 93 (17-726) | 73 (9-463) | NS |
| Liver volume, mL, median (range) | 3095 (1504-6542) | 1741 (1048-2821) | < .001 |
| Spleen volume, mL, median (range) | 1400 (470-4821) | 1597 (365-4195) | NS |
| BMB score, median (range) | 6.5 (0-8) | 7 (3-8) | NS |
| Chitotriosidase, nmol/mL/h, median (range) | 16 703 (5316-102 324) | 11 869 (592-39 791) | .056 |
| Patients with a serious bone complication in the 10 y prior to start of ERT, no. (%) | 21 (43) | 25 (44) | NS |
| Start dose, U/kg/4 wk, median (range) | 15 (15-50) | 80 (60-120) | < .001 |
| ERT cost per y for a 70-kg (154-lb) patient at start dose, median, US $ (€) | 69.85 (55.165) | 376.44 (294.221) | NA |
NA indicates not applicable; NS, not significant.
Chitotriosidase. Chitotriosidase activity in plasma samples from both centers were measured in one central laboratory at AMC. The standard enzyme activity assay with 4-MU-chitotriose (Sigma, St Louis, MO; normal range, 7-124 nmol/mL/h) as a substrate was performed at pH 5.2, as described previously.27 Genotyping for the chitotriosidase null mutation28 was performed, and chitotriosidase values of patients who were heterozygous for the chitotriosidase null mutation were multiplied by 2.29 For comparison of baseline values versus values after 12 months of ERT, patients with a chitotriosidase activity of more than 5000 nmol/mL/h were selected, and matched pairs were made according to baseline values (maximum baseline difference of 2500 nmol/mL/h). The time to reach a chitotriosidase activity of less than 5000 nmol/mL/h was determined in patients with baseline chitotriosidase activity in the range of 6000 nmol/mL/h to 25 000 nmol/mL/h.
Bone disease. For assessment of bone involvement, T1- and T2-weighted MRI images of the femora were used. Scoring of the severity of involvement of the bone marrow was adapted from the earlier described BMB.30 This scoring system correlates significantly to bone marrow fat fractions obtained with Quantitative Chemical Shift Imaging (QCSI), which has been shown to be closely related to clinical bone disease.31 The BMB score incorporates both the visual interpretation of signal intensity and the geographic location of the disease on conventional MR images of the spine and femur. Since images of the spine were not obtained at HHU, the scoring was limited to the femurs. All available MRIs of the femora were scored by a radiologist experienced in the evaluation of Gaucher patients, who was blinded for patient identity, treatment dosage, and time of assessment. The BMB score for the femur ranges from 0 (no abnormalities) to 8 points (severe disease). A change in BMB score adequately reflects reappearance of fatty marrow, indicating clearance of Gaucher cells. The time to reach a decrease of 2 BMB points from baseline was determined by life table analysis in all patients with a baseline BMB in the range of 2 to 8, and in a separate analysis for patients with severe bone disease. Severe bone disease was defined as a baseline BMB of at least 6, which is comparable to a QCSI less than 23%, a risk factor for serious bone complications.31 Results were presented as the share of patients who had reached the therapeutic goal after 24 months of ERT. The occurrence of skeletal complications, defined as new bone crisis, avascular necrosis, or pathologic fracture, during the 10 years prior to start of ERT or during the study period, was recorded from the patient files.
Results
Patient characteristics
Patient characteristics are presented in Table 1. Patients did not differ with respect to age, sex, or number of splenectomies, but some disease parameters were more severe in the AMC cohort. This was explained by the presence of several patients within the AMC cohort with extensive disease, especially patients with severe hepatomegaly after splenectomy (20 patients, 11 splenectomized, had liver volumes > 3000 mL), who were not found in the HHU cohort. This also was reflected in slightly lower hemoglobin levels and the presence of some patients with extremely high chitotriosidase levels in the AMC cohort. Oral bisphosphonates were used in none of the Duesseldorf patients and in 10% of the Dutch cohort, for a variable length of time.
In both patient groups, fewer than 10% of the patients were known to be of Ashkenazi-Jewish ancestry. The prevalence of the various mutations in both cohorts was comparable. Genotyping revealed that the most frequent mutation in both centers was N370S/L444P (37% and 32%). Homozygosity for N370S was found in 10% of Dutch and 9% of German patients, and absence of the N370S allele was found in none and in 3.5%, respectively.
The median time of treatment at AMC was 9 years (range, 1-12 years), and 7 years (range, 3-11 years) at HHU (P = .03). Adjustment of the dose, as described in “Patients and methods,” occurred especially at AMC; from 2 years onward the median dose at the AMC had increased to 30 U/kg/4 wks (range, 15-120 U/kg/4 wks), while the dose at HHU remained largely stable (median, 80 U/kg/4 wks; range, 60-120 U/kg/4 wks). At all time points, the dose remained significantly different (P < .001).
Disease parameters
There were no significant differences in age, sex, number of splenectomies, or SSI in any of the matched populations (data not shown).
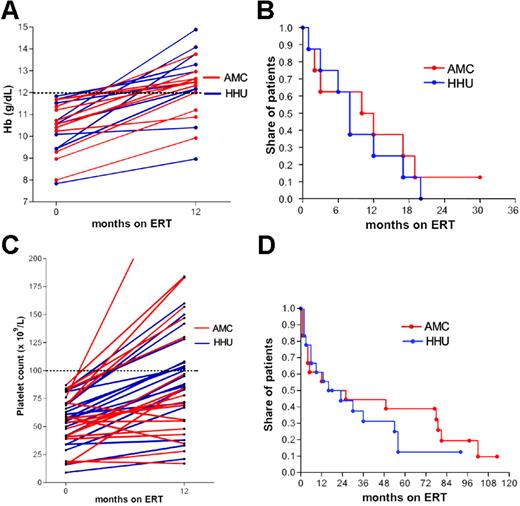
Hemoglobin and platelet count. Only 31% of the HHU cohort and 53% of the AMC cohort had anemia (Hb < 12.0 g/dL) at baseline. Matching for baseline hemoglobin of less than 12.0 g/dL and splenectomy status resulted in 2 groups of 11 patients. There was no significant difference in the increase of hemoglobin after 12 months of ERT (Figure 1A, P = .37). In 16 patients, follow-up data were available for life table analysis. The time to normalization was not significantly different between both cohorts (median time to normalization: 10 months for AMC, 8 for HHU; Figure 1B, P = .60). For all but one patient, hemoglobin normalized during ERT within 2 years.
In both cohorts, 58% of patients had thrombocytopenia. Matching for baseline platelet count less than 100 × 109/L and splenectomy status resulted in 2 groups of 19 patients. There were no significant differences in the increase in platelet count after 12 months of ERT (Figure 1C, P = .58). In 2 groups of 18 patients, follow-up data were available for life table analysis. The time to reach a value greater than 100 × 109/L was not significantly different between both cohorts (median time to > 100 × 109/L: 16 months for AMC, 16 for HHU, Figure 1D, P = .50).
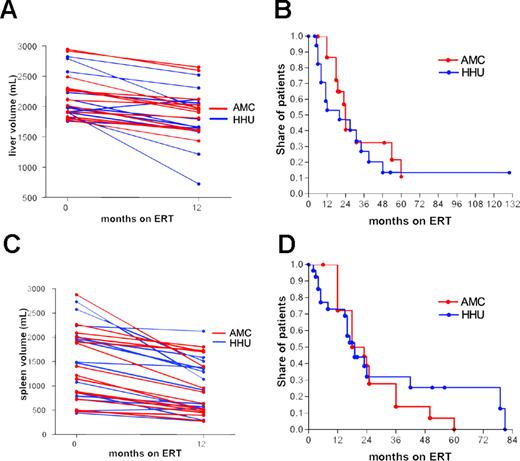
Liver and spleen volume. A total of 24 patients with hepatomegaly, defined as a liver volume greater than 1750 mL, were selected and matched according to baseline values and splenectomy status. Decrease in liver volume was not different in the 2 cohorts after 12 months of ERT (Figure 2A, P = .59).
Impact of ERT on changes in hemoglobin and platelet count. (A) Hemoglobin at baseline and after 12 months, (B) time to reach a hemoglobin count higher than 12 g/dL, (C) platelet count at baseline and after 12 months, (D) time to reach a platelet count higher than 100 × 109/L.
Impact of ERT on changes in hemoglobin and platelet count. (A) Hemoglobin at baseline and after 12 months, (B) time to reach a hemoglobin count higher than 12 g/dL, (C) platelet count at baseline and after 12 months, (D) time to reach a platelet count higher than 100 × 109/L.
Sixteen patients from AMC and 18 from HHU with a baseline liver volume between 1750 mL and 3000 mL were included in the life table analysis. There were no significant differences in the time to reach a decrease of 20% from baseline values (median time to decrease 20% from baseline: AMC, 24 months; and HHU, 20 months; Figure 2B, P = .32).
A total of 28 patients with a spleen volume greater than 250 mL was selected and matched according to baseline values. There were no significant differences in the decrease of spleen volume after 12 months of ERT (Figure 2C, P = .84).
Nineteen patients from AMC and 27 from HHU with a baseline spleen volume of 400 mL to 2300 mL were included in the life table analysis. There was no significant difference in time to reach a decrease of 40% from baseline (median time to decrease 40% from baseline: AMC, 18 months; and HHU, 19 months; Figure 2D, P = .69).
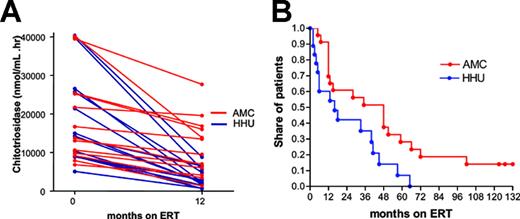
Chitotriosidase. Four patients were excluded because of homozygosity for the chitotriosidase null mutation. Twenty-seven patients were carriers of the chitotriosidase null mutation and had their levels multiplied by 2.29 Matching of patients with a chitotriosidase activity higher than 5000 nmol/mL/h resulted in 2 groups of 13 patients. The decrease in chitotriosidase activity after 12 months of ERT was significantly stronger in the HHU cohort than in the AMC cohort (Figure 3A, P = .001). Twenty-three patients from AMC and 18 patients from HHU with a baseline chitotriosidase level of 6000 to 25 000 nmol/mL/h were included in the life table analysis. There was a significant difference between both cohorts in time to reach a value of less than 5000 nmol/mL/h (median time to < 5000 nmol/mL/h: AMC, 48 months; and HHU, 16 months; Figure 3B, P = .015). All patients from HHU reached a chitotriosidase level lower than 5000 nmol/mL/h, while 15% of the AMC cohort did not.
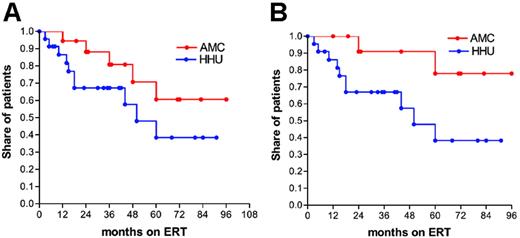
Bone disease. Comparison of the time to reach a decrease of 2 points in BMB score from baseline, for patients with a baseline BMB of 2 to 8, showed a trend toward a quicker response in the HHU cohort, with 12% of patients from AMC (n = 19) reaching the therapeutic goal after 24 months of ERT versus 33% of patients from HHU (n = 23, Figure 4A, P = .11).
Impact of ERT on changes in liver and spleen volume. (A) Liver volume at baseline and after 12 months, (B) time to reach a 20% decrease of liver volume from baseline, (C) spleen volume at baseline and after 12 months, (D) time to reach a 40% decrease of spleen volume from baseline.
Impact of ERT on changes in liver and spleen volume. (A) Liver volume at baseline and after 12 months, (B) time to reach a 20% decrease of liver volume from baseline, (C) spleen volume at baseline and after 12 months, (D) time to reach a 40% decrease of spleen volume from baseline.
Impact of ERT on changes on plasma chitotriosidase activity. (A) Plasma chitotriosidase at baseline and after 12 months, (B) time to reach a chitotriosidase of less than 5000 nmol/mL/h. Chitotriosidase levels of carriers of the chitotriosidase null mutation were multiplied by 2.29
Impact of ERT on changes on plasma chitotriosidase activity. (A) Plasma chitotriosidase at baseline and after 12 months, (B) time to reach a chitotriosidase of less than 5000 nmol/mL/h. Chitotriosidase levels of carriers of the chitotriosidase null mutation were multiplied by 2.29
Subanalysis of patients with more severe bone disease (BMB ≥ 6) resulted in a significant difference (Figure 4B, P = .04). After 24 months of ERT, 10% of patients from the AMC cohort (n = 13) reached a decrease of 2 points BMB from baseline versus 33% of the HHU cohort (n = 22).
Analysis of the clinical records showed that, during the 10 years before start of ERT, the number of patients with a serious bone complication was comparable (43% in AMC, 44% in HHU). During ERT skeletal complications occurred in 2 patients from AMC (a mild bone crisis after trauma and an avascular necrosis of the left head of the femur without any clinical symptoms, detected by routine MRI) and one patient from HHU (pathologic fracture of the left toe V) (P = .47). Atypical bone pain persisted in some patients from both groups but was not analyzed systematically.
Discussion
Because ERT for type 1 Gaucher disease is extremely expensive, the long-standing controversy about relative efficacy of high-dose versus low-dose treatment regimens has great socioeconomic as well as medical significance. Comparative studies have been limited. Beutler19 performed a meta-analysis on several disease parameters from published cohorts and showed no difference in response. However, parameters on bone disease and biochemical markers were not available for analysis. One prospective trial compared doses of 10 U/kg/2 wks to the “standard” dose of 60 U/kg/2 wks.13 Although it was shown that the low dose was less effective with respect to improvement in hemoglobin values and liver and spleen volumes, the patient populations were not matched for disease severity, and skeletal parameters as well as biochemical parameters were lacking. In this retrospective study, we compared the response of all major disease parameters, including bone disease parameters and chitotriosidase values, in a large number of adult Gaucher type 1 patients from AMC and HHU who were treated for up to 12 years with a relatively low (AMC) and relatively high dosage (HHU) of ERT. Both populations were similar with respect to age, sex, genotypes, and splenectomy status. However, because several severely affected patients in the AMC cohort had no counterparts in the HHU cohort, patients were matched for baseline values of the separate parameters. This resulted in a reduction of the size of the parameter-specific cohorts, but allowed a more accurate analysis.
Impact of ERT on changes in bone marrow burden score. (A) Time to reach a decrease of 2 points in BMB score, as measured by MRI, from baseline of patients with a baseline BMB of 2 to 8, (B) time to reach a decrease of 2 points in BMB score from baseline of patients with a baseline BMB of 6 to 8.
Impact of ERT on changes in bone marrow burden score. (A) Time to reach a decrease of 2 points in BMB score, as measured by MRI, from baseline of patients with a baseline BMB of 2 to 8, (B) time to reach a decrease of 2 points in BMB score from baseline of patients with a baseline BMB of 6 to 8.
We found that improvement of hemoglobin, platelet count, and liver and spleen volume is not dose dependent. However, chitotriosidase does respond better to a relatively high initial dose. Improvement in BMB, an MRI-based scoring system of bone marrow involvement, shows a strong trend toward a better response to higher doses, particularly in patients with more extensive bone marrow disease in whom the difference is statistically significant.
For all parameters, except for bone disease, 2 separate statistical methods were used to assess dose responsiveness. Life table analysis is good for depicting long-term changes but is influenced by the length of the interval between consecutive samples. This is relevant because the HHU cohort was followed at less frequent intervals than the AMC cohort, possibly leading to an underestimation of the responses in the patients treated with higher-dose ERT. However, this is unlikely, because of the alternate analysis, in which baseline values were compared to values after one year of therapy, which is independent of the interval between samples. Both methods showed similar outcomes: dose independence for anemia, thrombocytopenia, and organomegaly and dose dependence for chitotriosidase and bone marrow burden.
Whether the higher initial frequency at AMC could have beneficially influenced the outcome is unknown. Zimran et al21 showed no difference in a 3-times-a-week schedule compared to a fortnightly administration of imiglucerase, but Altarescu et al13 applied a low dose of 20 U/kg/4 wks in a fortnightly schedule with inferior responses, compared in retrospect to data obtained from the literature with even lower doses given at high frequency.12,20 The current study cannot give an answer to this issue, but it is very likely that, should there be an effect at all, the differences between the cohorts with respect to response in chitotriosidase and BMB would have been even more pronounced if the low dose was given at low frequency.
Chitotriosidase is a lysosomal enzyme that originates from Gaucher cells and is closely associated with total body burden of Gaucher cells.27 It is increased 100- to more than 4000-fold in symptomatic patients, while it is not or only slightly increased in asymptomatic patients. Several studies also have shown a relationship between parameters of disease burden such as SSI and organ volumes and chitotriosidase levels.32,33 Its use as a biomarker is limited by the observation that 6% of the population lacks activity and 30% carries a mutation that results in lower activities.34 In heterozygote patients, chitotriosidase levels may be multiplied by 2 to allow adequate comparison with Gaucher patients with normal chitotriosidase genotypes.29 The clinical usefulness of chitotriosidase has been the subject of a limited number of studies. First of all it was documented that in patients after cessation of treatment, chitotriosidase impressively precedes deterioration of clinical symptoms.35 It also was established that patients who did not reach a decrease in chitotriosidase of 15% from baseline within the first 12 months also had a lack of clinical response.36 These data provide an indication that the persistence of high chitotriosidase levels reflect the presence of a high burden of Gaucher cells. Whether this can be translated into an increased risk for Gaucher-related morbidity remains to be determined.
Of all disease parameters, bone disease is the most difficult to evaluate. MR imaging is the preferred modality, due to its sensitivity for the detection of both focal and diffuse disease.37-39 Ideally, quantification is done by fat fraction measurements of the lumbar spine obtained through the use of Dixon QCSI,40,41 which shows a close correlation with the occurrence of clinical complications.31 However, since QCSI is not widely available and vertebral MRI was not performed in the HHU cohort, in this study, we resorted to use the BMB30 of the femora, a semiquantitative scoring system that is significantly correlated to QCSI.
The decision to use the modified BMB score may have affected our results, because in contrast to the BMB score of the axial skeleton, small changes in bone disease are difficult to recognize in the femora due to irreversible changes such as marrow infarction or avascular necrosis that are more common in the lower extremities than in the spine. A potential bias lies in the larger number of MRI examinations in the HHU (high-dose) patients relative to the AMC (low-dose) patients, which may exaggerate the difference in response between relatively low and high dosing. However, the median time interval between examinations was not very different (12.2 months for AMC; 14.9 months for HHU), and analysis of the data at a fixed time interval, between baseline and 12 months, showed the same results: a trend toward better response to high dosing for the whole group, which was significant in patients with a BMB of at least 6 (P = .03). The restriction of MRI examinations to the femora also may explain why, aside from any purported dose effect, favorable hematologic and organ responses do not necessarily correlate with a good bone marrow response. In fact, although most patients achieved hematologic and organ therapeutic goals, even among the HHU (high-dose) patients a significant proportion failed to achieve the BMB therapeutic goal. Whether failing to reach the therapeutic goal for BMB is associated with a higher risk of severe bone complications cannot be answered with this study, since the number of events during ERT was low in both groups (2 for AMC, 1 for HHU). Whether the groups differed with respect to chronic bone complaints is uncertain, since these data were not collected systematically. Nevertheless, the clearly established identification of low fat in the bone marrow as a risk factor for skeletal complications31 points toward a clinically relevant difference in favor of high dosing in patients with severe bone disease, at least in the initial treatment period. This conclusion is supported by the observation that both patients from AMC who developed posttreatment skeletal complications had no history of prior skeletal events but did have persistently high BMB scores while on low-dose ERT.
Based on the results of this study, the following recommendations can be made. First, as improvement of hemoglobin, platelet count, and liver and spleen volume is not dose dependent, extensive organomegaly and cytopenia do not justify a high initial dose. Severe bone marrow involvement, on the other hand, may carry a risk for bone complications and is an important criterion to start a higher dose of enzyme. Nevertheless, because the number of major bone complications was low in both groups, this hypothesis merits further investigation. The determination of the most cost-effective dosing regimen should be made individually and on the basis of a complete disease profile, including proper assessment of bone marrow involvement in addition to hematological, visceral, and biochemical parameters. This should be done not only for initiation and monitoring of ERT, but also in future studies on ERT as well as alternative treatments for Gaucher disease such as chaperone-based therapies or substrate reduction. Chitotriosidase proves to be a sensitive indicator of dose effects and may be used in that respect to monitor response. Subsequent follow-up of all disease parameters is mandatory to allow adequate dosing and appropriate dose adjustment.
Prepublished online as Blood First Edition Paper, March 9, 2006; DOI 10.1182/blood-2005-12-5072.
Supported in part by the governmental funding of the Academic Medical Center for the centralized treatment and monitoring of Gaucher disease patients in The Netherlands. Travel costs were in part supported by the Federal Bureau for Advancing the Relationships between Universities of North-Rhine-Westphalia (Germany) and the Universities of the Benelux countries.
M.d.F. participated in designing the study, analyzing the data, and writing the report; C.E.M.H. and S.v.D. participated in designing and supervising the study and writing the report; J.E.M.G. participated in the biochemical analysis; J.M.F.G.A. participated in supervising the study and writing the report; M.M. participated in collecting and analyzing radiologic data and scoring MRIs; L.W.P. participated in collecting and analyzing radiologic data; M.G.W. participated in collecting the Dutch clinical data; D.H. participated in writing the report; and S.B. and N.B. participated in collecting the German clinical data.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
C. H. is a member of the medical board that assists in providing access to cerezyme in Eastern European countries, led by Genzyme, for which she does not receive any payment apart from reimbursement for travel expenses.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal