Comment on Martino et al, page 836
In this issue of Blood, Martino and colleagues present a retrospective analysis showing that survival after RIC allogeneic SCT is similar to conventional myeloablative allogeneic SCT for patients with MDS and secondary AML despite treatment of higher risk and older patients.
It has long been accepted that allogeneic stem cell transplantation (SCT) is successful in part due to the graft-versus-leukemia (GVL) effect provided by donor T cells, identified indirectly from retrospective analyses,1 and directly through the use of donor lymphocyte infusions (DLIs) to treat relapse after allogeneic SCT.2,3 This provides the rationale for the explosion of reduced-intensity conditioned (RIC) transplantations in the past several years. RIC SCT attempts to deliver sufficient immunosuppression to allow donor cell engraftment while minimizing conditioning regimen toxicity. The promise of RIC SCT is to improve cure rates by lowering treatment-related mortality (TRM) and to rely primarily on GVL induction to eradicate malignant cells.
In conventional allogeneic SCT, almost uniformly, manipulations that limit graft-versus-host disease (GVHD) are offset by higher relapse rates, and treatments that enhance GVL are offset by increased mortality from GVHD. Manipulation of conditioning regimen intensity leads to a different but equally frustrating balance between the need for cytotoxicity and the TRM associated with dose-intensive therapy.
The article by Martino and colleagues highlights this fine balance. This retrospective analysis compares outcomes after matched-sibling transplantation in 215 recipients of RIC regimens to 621 recipients of standard myeloablative conditioning (SMC). As might be anticipated, the lower-intensity regimens led to less TRM but higher relapse, while SMC resulted in fewer relapses but higher TRM. The lower TRM after RIC was largely offset by the higher relapse rate leading to similar 3-year progression-free survival (PFS) and overall survival in both groups (39% after SMC vs 33% after RIC; P = .8). This balance between high relapse rates after RIC and TRM after SMC has been noted by others.4
Patient selection for conventional and RIC transplantation is likely to be very different, and even the conditioning regimen choice may influence outcomes. Higher-dose RIC regimens will likely minimize relapse for some diseases,5 while more immunosuppressive GVHD prophylaxis could limit response or increase relapse risk.6 In this study, recipients of RIC were older than recipients of SMC (median age, 56 years vs 45 years). However, many factors other than age lead to a decision to undergo RIC rather than SMC. The international prognostic scoring system (IPSS) was not available in most cases, and it is not obvious that RIC recipients had higher-risk disease. In fact, at many centers, RIC is reserved for patients with less advanced disease.
The authors discuss the limitations of this type of retrospective study. Importantly, one cannot determine how regimen intensity was chosen for a particular patient, comorbidities and prior therapies are not documented, and a number of disease- and conditioning regimen–related variables are not reported. For instance, in the RIC patients alone, at least 7 different conditioning regimens and 4 GVHD prophylactic therapies are listed that could account for 28 different combinations. All these issues make it difficult to compare these patient populations. To clarify these concerns, the authors are launching a prospective randomized trial comparing SMC with a homogeneous RIC regimen, and appropriately recommend that RIC transplantation be reserved for patients ineligible for conventional allogeneic SCT.FIG1
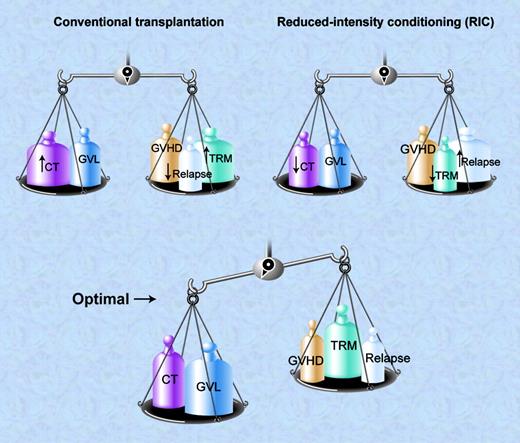
Conventional allogeneic SCT balances high-dose conditioning therapy (CT) and GVL effects against GVHD, high TRM, but low relapse rates. RIC uses lower dose CT and relies heavily on GVL activity balanced against the potential for higher relapse rates and GVHD but lower direct TRM. The optimal balance occurs when the combination of CT and GVL outweighs the risk of relapse, GVHD and TRM. Illustration by A. Y. Chen.
Conventional allogeneic SCT balances high-dose conditioning therapy (CT) and GVL effects against GVHD, high TRM, but low relapse rates. RIC uses lower dose CT and relies heavily on GVL activity balanced against the potential for higher relapse rates and GVHD but lower direct TRM. The optimal balance occurs when the combination of CT and GVL outweighs the risk of relapse, GVHD and TRM. Illustration by A. Y. Chen.
That overall survival is not different after RIC compared with SMC, despite lower TRM, might suggest that RIC has not led to significant progress. However, because of patient selection, it is very likely that recipients of RIC would experience high TRM from SMC. This should lead to continued optimism that when it comes to RIC allogeneic SCT for MDS, this glass is half full. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal