We read the letter by Grogg et al,1 recently published in Blood, with great interest. The authors demonstrated that CD10+ T cells in angioimmunoblastic T-cell lymphoma2 (AITL) coexpress CXCL13 and proposed that AITL is a neoplasm of germinal center (GC) T-helper cells.
We have hypothesized that neoplastic T cells may be related to follicular B helper T (TFH) cells,3-6 since CD10+ atypical T cells were found to be in intimate contact with the expanded meshwork of proliferating follicular dendritic cells (FDCs), a characteristic of AITL. Therefore, we have performed consecutive double immuno-labeling to analyze expression of some known TFH cell functional antigens (CXCR5, CD154/CD40L, CD134/OX40, and CD57) in the CD10+ T cells in 20 paraffin-embedded AITL cases. Since Kim et al7 and Chtanova et al8 established that CXCL13 is highly up-regulated in TFH cells, expression of this antigen was also investigated; the same has also been done by Grogg et al.1
In 18 of 20 cases, the expression of CD10 on 5% to 30% of T cells strongly correlated with CXCL13 production. The remaining 2 cases exhibited CXCL13+ atypical T cells without apparent CD10 expression. The neoplastic T cells, as defined by CD10 as well as CXCL13 expression, revealed a CXCR5+CD134+CD154+ CD57–/+ immunophenotype (Figure 1), which is most consistent with GC outer-zone TFH cells.6 All cases displayed concomitant expression of CXCL13 and CXCR5 in neoplastic T cells, again pointing to a TFH phenotype.3-8 Coexpression of the CXCR5 chemokine receptor and its ligand CXCL13 suggests an autocrine loop, possibly contributing to the survival of neoplastic T cells. All cases demonstrated coexpression of CD154 in the majority of the neoplastic T cells. This antigen plays a crucial role in GC formation9 and provides survival signals to follicular B cells.6 Each case showed significant but variable numbers (10%-100%) of CXCL13+ T cells bearing CD134, whose expression on activated CD4+ T cells either initiates their migration into or causes them to be retained in B follicles.10 One case coexpressed CD57 in a significant proportion of neoplastic cells, while the remaining cases displayed only a minute fraction of CD57+CXCL13+ cells that may reflect the residual normal TFH cells. Since proliferating FDCs expressed CXCL13 and are known to express CD409 (receptor for CD154) and OX40L6,10 (ligand for CD134), our data suggest a selective cross-talk between FDCs and neoplastic T cells.
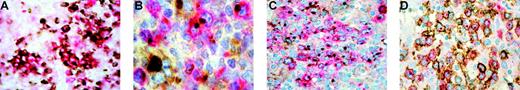
Activated follicular B helper T-cell phenotype of neoplastic cells in AITL. (A) Double immunostaining for CD10 in brown (DAB) and CXCL13 in red (Fast red TR) shows coexpression of CD10 and CXCL13 in tumor cells (no counterstain). (B) Double immunostaining for CXCL13 in red (Fast red TR) and CXCR5 in brown (DAB). The CXCL13+ neoplastic cells display concomitant positivity for CXCR5. (C) Double immunostaining for CXCL13 in red (Fast red TR) and CD154 in brown (DAB) that demonstrates overlaying cytoplasmic staining in the neoplastic cells. (D) Double immunostaining for CD134 in brown (DAB) and CXCL13 in red (Fast red TR) shows coexpression of these 2 antigens in the neoplastic cells. Original magnifications, × 400 (A,C-D) and × 1000 (B). The double stainings were performed using EnVision-HRP (A,D) or CSA II System (B-C) in combination with EnVision-AP (A-D; all from DakoCytomation, Carpinteria, CA). Primary antibodies were as follows: polyclonal goat anti-CXCL13 (A-D) and monoclonal mouse anti-CXCR5 (B; R&D Systems, Minneapolis, MN); monoclonal mouse anti-CD10 (A), anti-CD154 (C), and anti-CD134 (D; Novocastra Laboratories, Newcastle upon Tyne, United Kingdom). Images were visualized on a Nikon Eclipse E600 microscope equipped with a Nikon Plan 40 ×/0.65 objective or 100 ×/1.25 oil-immersion objective lens; images were then captured via a Nikon Coolpix 4500 digital camera and processed with Adobe PhotoShop 7.0 software (Adobe Systems, San Jose, CA).
Activated follicular B helper T-cell phenotype of neoplastic cells in AITL. (A) Double immunostaining for CD10 in brown (DAB) and CXCL13 in red (Fast red TR) shows coexpression of CD10 and CXCL13 in tumor cells (no counterstain). (B) Double immunostaining for CXCL13 in red (Fast red TR) and CXCR5 in brown (DAB). The CXCL13+ neoplastic cells display concomitant positivity for CXCR5. (C) Double immunostaining for CXCL13 in red (Fast red TR) and CD154 in brown (DAB) that demonstrates overlaying cytoplasmic staining in the neoplastic cells. (D) Double immunostaining for CD134 in brown (DAB) and CXCL13 in red (Fast red TR) shows coexpression of these 2 antigens in the neoplastic cells. Original magnifications, × 400 (A,C-D) and × 1000 (B). The double stainings were performed using EnVision-HRP (A,D) or CSA II System (B-C) in combination with EnVision-AP (A-D; all from DakoCytomation, Carpinteria, CA). Primary antibodies were as follows: polyclonal goat anti-CXCL13 (A-D) and monoclonal mouse anti-CXCR5 (B; R&D Systems, Minneapolis, MN); monoclonal mouse anti-CD10 (A), anti-CD154 (C), and anti-CD134 (D; Novocastra Laboratories, Newcastle upon Tyne, United Kingdom). Images were visualized on a Nikon Eclipse E600 microscope equipped with a Nikon Plan 40 ×/0.65 objective or 100 ×/1.25 oil-immersion objective lens; images were then captured via a Nikon Coolpix 4500 digital camera and processed with Adobe PhotoShop 7.0 software (Adobe Systems, San Jose, CA).
Our observations not only fully support the notion of Grogg et al1 but extend it by a more detailed analysis, establishing that the phenotype of the neoplastic cells (as shown here) is consistent with activated TFH cells localized at the boundary between the mantle zone and the GC light zone.6 These findings provide direct explanations for some peculiar features of AITL, including B-cell hyperactivation and hypergammaglobulinemia despite gradual reduction of follicular B-cell mass, as well as the follicular outgrowths of FDCs as a result of stimulation by neoplastic TFH cells. Further investigations will be needed to explain loss of follicular B cells, a phenomenon that occurs in advanced cases and may be caused by a disarranged dialogue between neoplastic TFH cells and nonneoplastic B cells.
L.K. designed research, performed research, analyzed data, and wrote the paper, P.S. analyzed data and wrote the paper; G.K. performed research; and E.B. performed research and analyzed data.
Supported by the Hungarian Scientific Research Fund (OTKA -T 046663 KON).
The cases studied were obtained from the Hungarian T-Cell Lymphoma Register.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal