Abstract
The diagnosis of myelodysplastic syndromes (MDS) without an increase in blasts and ringed sideroblasts (low-grade MDS without ringed sideroblasts [LGw/oRS]) may be problematic because dysplastic features are not specific to MDS and approximately 50% of patients with LGw/oRS lack chromosomal aberrations. Here, we report the usefulness of flow cytometric characteristics of CD34+ cells for LGw/oRS diagnosis. Bone marrow cells from LGw/oRS patients and controls (eg, cytopenic individuals without MDS) were analyzed using 4-color flow cytometry (FCM). We objectively determined reference ranges of 13 parameters related to CD34+ cells with data from controls. In LGw/oRS patients, various abnormalities of CD34+ cells—eg, decrease in CD34+ B-cell precursors, aberrant expression or overexpression of various antigens on CD34+ myeloblasts—were observed. We constructed a reproducible, flow cytometric scoring system for LGw/oRS diagnosis. High scores were observed in 16 of 27 LGw/oRS patients, regardless of the presence or absence of chromosomal aberrations, but not in any of the 90 controls. Among LGw/oRS patients with chromosomal aberrations, patients with trisomy 8 or del20(q) had low FCM scores (P = .002). As a result, most LGw/oRS patients were identified based on high FCM score, chromosomal aberration, or both.
Introduction
Myelodysplastic syndromes (MDSs) are malignant disorders of hematopoietic cells that typically develop in elderly persons and for which the prognosis is usually poor.1,2 In patients with MDSs, peripheral blood (PB) is usually cytopenic (or rarely shows an increase in blood cells3,4 ), and bone marrow (BM) is composed of clonal hematopoietic cells showing varying degrees of differentiation. The diagnosis of MDS has been made based on a combination of clinical history, morphologic features of PB and BM (eg, percentages of blasts and dysplastic features of cells), and cytogenetic data and by ruling out other diseases. Refractory anemia (RA) as originally defined by the French-American-British (FAB) cooperative group5 is one subtype of MDS that accounts for 20% to 40% of all MDS cases.6 The recent World Health Organization (WHO) classification refined the definition of dysplasia for MDS diagnosis and subcategorized the original RA into “RA,” “refractory cytopenia with multilineage dysplasia (RCMD),” “MDS, unclassified (MDS-U),” and “MDS associated with isolated del(5q).”7 The diagnoses of RA, RCMD, and MDS-U (designated in this paper as low-grade MDS without ringed sideroblasts [LGw/oRS]) are associated with several difficulties. LGw/oRS does not exhibit an increase in blasts and ringed sideroblasts over the normal range; blasts and ringed sideroblasts are clearly objective cytologic findings observed in other MDS subtypes. LGw/oRS lacks cytogenetic abnormalities in 50% or more of patients.8,9 The dysplastic features of hematopoietic cells do not in themselves establish a diagnosis.10 Conditions other than MDS can induce dysplastic hematopoietic cells (eg, vitamin B12 and folate deficiencies, viral infections, and exposure to antibiotics, chemotherapeutic agents, ethanol, benzene, or lead) and should be ruled out by careful history taking and physical and laboratory examinations.
Immunophenotyping using flow cytometry (FCM) is an objective and reliable method to identify dysregulated antigen expression of neoplastic cells. Recent studies have suggested that the abnormalities of hematopoietic cells detected by FCM might be useful for a diagnosis of MDS.11-14 Those studies used a pattern recognition approach: based on extensive knowledge of the normal pattern of hematopoietic cells in FCM, investigators identified abnormal patterns (such as hypogranulation of neutrophils by orthogonal light scatter) without establishing an objective definition of the abnormal pattern. Therefore, the usefulness of this approach was debated,15,16 and the diagnostic usefulness of FCM for MDS has not yet been widely accepted. The blasts in acute myeloid leukemia often show dysregulated antigen expression, which has been used to detect residual disease.17,18 Recently, we immunophenotyped blasts from 95 patients with various MDS subtypes and found that a high proportion of blasts from almost all were CD34+CD13+CD33+.19 We also found that MDS blasts often exhibit aberrant antigen expression.19
We examined 13 flow cytometric parameters of CD34+ cells from LGw/oRS patients and controls, including patients who should be differentiated clinically from those with LGw/oRS (eg, cytopenic patients with conditions other than MDS). By comparing data between patients and controls, we clarified the broad range of CD34+ cell–related abnormalities in LGw/oRS patients. Furthermore, using the data from controls, we determined reference ranges (RRs) of the 13 parameters and constructed a flow cytometric scoring system for LGw/oRS diagnosis. High scores were exclusively associated with LGw/oRS patients who had or did not have chromosomal aberrations. We also found that, among LGw/oRS patients with chromosomal aberrations, patients with trisomy 8 or del20(q) had low FCM scores and that most LGw/oRS patients had high FCM scores, chromosomal aberrations, or both.
Patients, materials, and methods
Patients
We studied 2 groups of consecutive patients who underwent BM aspiration: new patients with abnormal PB findings (eg, cytopenia and macrocytosis) for whom clinicians considered LGw/oRS a possible diagnosis, and patients in whom LGw/oRS or lymphoma had been diagnosed and who underwent BM examination for evaluation of disease status.
Wright-Giemsa–stained smears of the aspirated BM cells were prepared for every patient. If glancing at the smears (rough screening) obviously showed high-grade MDS (RA with excess blasts [RAEB] and RAEB in transformation [RAEB-t]) or other malignancies (eg, acute leukemia, myeloma, BM invasion by lymphoma or carcinoma), BM samples were excluded from this study. After written informed consent had been obtained from the patients, other BM samples were sent to a laboratory for the present 4-color FCM designed for LGw/oRS diagnosis. These samples were also analyzed in conventional laboratory tests, which included cytologic and pathologic examinations, cytogenetic analysis according to standard G-banding technique, and, for lymphoma patients, FCM with the appropriate antibodies to detect lymphoma cell invasion.
Patients were divided into 2 separate cohorts (Table 1). The first cohort included the initial 75 patients whose BM was analyzed with 4-color FCM (12 LGw/oRS patients, 50 controls, and 13 excluded patients who had passed the rough screening but were later found to be unsuitable as controls, such as those diagnosed with high-grade MDS and other neoplastic diseases with BM invasion). Based on the flow cytometric data of these LGw/oRS patients and controls, we defined flow cytometric diagnostic criteria for LGw/oRS. We then continued to use 4-color FCM to analyze the BM of subsequent patients (the second cohort), and we examined the validity of the flow cytometric diagnostic criteria for LGw/oRS.
Consecutive patients and controls analyzed using 4-color FCM
Diagnosis . | No. in each cohort . |
|---|---|
| First cohort | |
| LGw/oRS patients (no. male/no. female) | 12 (7/5) |
| Controls (no. male/no. female) | 50 (24/26) |
| ITP | 14 |
| NHL without BM invasion | 10 |
| Anemia of chronic disease* | 7 |
| Healthy, marginal cytopenia† | 6 |
| HD without BM invasion | 3 |
| Iron-deficiency anemia | 2 |
| Alcoholic cytopenia or macrocytosis | 2 |
| Drug-induced anemia | 1 |
| Chronic obstructive lung disease | 1 |
| Renal failure | 1 |
| Infectious mononucleosis | 1 |
| Thalassemia | 1 |
| Pure white cell aplasia | 1 |
| Second cohort | |
| LGw/oRS patients (no. male/no. female) | 15 (10/5) |
| Controls (no. male/no. female) | 40 (21/19) |
| ITP | 9 |
| Healthy†‡ | 8 |
| NHL without BM invasion | 7 |
| Drug-induced cytopenia | 4 |
| Anemia of chronic disease* | 3 |
| Vitamin B12 deficiency | 2 |
| Renal failure | 2 |
| Alcoholic cytopenia | 1 |
| Chronic obstructive lung disease | 1 |
| Pure red cell aplasia | 1 |
| ATP | 1 |
| Hypothermia-induced cytopenia | 1 |
Diagnosis . | No. in each cohort . |
|---|---|
| First cohort | |
| LGw/oRS patients (no. male/no. female) | 12 (7/5) |
| Controls (no. male/no. female) | 50 (24/26) |
| ITP | 14 |
| NHL without BM invasion | 10 |
| Anemia of chronic disease* | 7 |
| Healthy, marginal cytopenia† | 6 |
| HD without BM invasion | 3 |
| Iron-deficiency anemia | 2 |
| Alcoholic cytopenia or macrocytosis | 2 |
| Drug-induced anemia | 1 |
| Chronic obstructive lung disease | 1 |
| Renal failure | 1 |
| Infectious mononucleosis | 1 |
| Thalassemia | 1 |
| Pure white cell aplasia | 1 |
| Second cohort | |
| LGw/oRS patients (no. male/no. female) | 15 (10/5) |
| Controls (no. male/no. female) | 40 (21/19) |
| ITP | 9 |
| Healthy†‡ | 8 |
| NHL without BM invasion | 7 |
| Drug-induced cytopenia | 4 |
| Anemia of chronic disease* | 3 |
| Vitamin B12 deficiency | 2 |
| Renal failure | 2 |
| Alcoholic cytopenia | 1 |
| Chronic obstructive lung disease | 1 |
| Pure red cell aplasia | 1 |
| ATP | 1 |
| Hypothermia-induced cytopenia | 1 |
ITP indicates immune thrombocytopenic purpura; NHL, non-Hodgkin lymphoma; HD, Hodgkin disease; ATP, amegakaryocytic thrombocytopenic purpura.
Ages were 61.9 ± 17.1 and 60.6 ± 17.4 years for LGw/oRS patients and controls, respectively, in the first cohort and 74.9 ± 8.6 and 61.1 ± 15.1 years for LGw/oRS patients and controls, respectively, in the second cohort.
First cohort: 3 congestive heart failure, 2 carcinoma without BM invasion (1 lung cancer, 1 pancreatic cancer), 1 fever of unknown origin, 1 undefined inflammation. Second cohort: 1 sarcoidosis, 1 rheumatoid arthritis, 1 pyoderma gangrenosum.
Patients who had marginal cytopenia or neutrophilia and were judged to be healthy after examination.
Seven marginal cytopenias and 1 physiologic.
Among the excluded patients in the first cohort, one had RAEB, one had RA with ringed sideroblasts (RARS), 2 had chronic myeloid leukemia (CML), 4 had aplastic anemia (AA), and 3 had non-Hodgkin lymphoma (NHL) with BM invasion. For 2 patients, clinical information was insufficient. In the second cohort, the excluded patient group consisted of one with RAEB, one with RARS, one with CML, 2 with essential thrombocythemia, 5 with AA, one with NHL with BM invasion, and one with insufficient clinical information. Two NHL patients for whom sufficient numbers of BM cells were unavailable were also excluded. We did not exclude patients with MDS associated with isolated del(5q), but our cohorts did not contain any such patients, because this disease is rare in the Japanese population.20
MDS was diagnosed independently by 2 hematologists, both experienced in MDS diagnosis, in accordance with WHO criteria.7 They examined 500 BM nucleated cells and 100 PB nucleated cells in well-prepared Wright-Giemsa–stained smears, the cytochemistry of BM smears, and histologic sections of BM from all patients. Dysplasia was judged to be present in a lineage if 10% or more of BM nucleated cells of the corresponding lineage were dysplastic. Other diseases were ruled out by repeated history taking and physical and laboratory examinations, including follow-up PB data. Both hematologists differed in their initial diagnoses for one patient, so smears and other data were reexamined by them jointly and a consistent diagnosis was obtained. Karyotypes were interpreted according to International System for Cytogenetic Nomenclature criteria.21 Two patients who had cytopenia (and/or macrocytosis) and morphologically normal BM cells but clonal cytogenetic aberrations were included among the LGw/oRS patients. The International Prognostic Scoring System (IPSS) was applied to LGw/oRS patients according to a previous report.22 This study protocol was approved by the institutional review board of Nippon Medical School.
Four-color FCM
BM cells of the patients were aspirated into a heparinized syringe, immediately diluted 2-fold with RPMI 1640 medium containing 10% fetal calf serum, and stored at 4°C overnight. This temperature was chosen because FCM data do not differ between samples stored at 4°C and room temperature (approximately 16°C-28°C), but the data of B cells become unstable at higher temperatures.23 The next morning (19-24 hours after cell aspiration), erythrocytes were lysed using the standard ammonium chloride method and were washed with phosphate-buffered saline, and nucleated cells were counted using a Coulter counter. Cell suspensions were divided into 100-μL aliquots, each of which contained 3 × 105 nucleated cells, and were stained with anti-CD45 antibody conjugated with peridin chlorophyll (PerCP) (Becton Dickinson [BD], Franklin Lakes, NJ), anti-CD34 antibody conjugated with allophycocyanin (APC) (isotype IgG1; BD), and pairs of antibodies conjugated with either fluorescein isothiocyanate (FITC) or phycoerythrin (PE). FITC-labeled antibodies were used against CD2 (IgG2a; BD), CD10 (IgG2a; BD), CD56 (IgG2b; BD), and CD15 (IgM; BD), and PE-labeled antibodies were used against CD4 (IgG1; BD), CD123 (IgG1; BD), CD11b (IgG2a; BD), CD13 (IgG1; BD), CD33 (IgG1; BD), CD117 (IgG1; BD), and CD133 (IgG1; Miltenyi Biotec, Bergisch Gladbach, Germany). These antibodies were chosen based on our previous report19 and on results of preliminary experiments, and the optimal volume of each antibody reagent for the staining was determined beforehand.
Isotype-matched negative controls for APC-, FITC-, and PE-conjugated antibodies (IgG1-APC [BD], IgG2a-FITC [BD], IgG2b-FITC [PharMingen, San Diego, CA], IgM-FITC [PharMingen], IgG1-PE [BD], and IgG2a-PE [BD]) were used in all assays. Data were acquired using a FACSCalibur flow cytometer (BD). Single-labeled cells were used to compensate for fluorescence emission overlap of each fluorochrome into inappropriate channels. At least 100 000 cell events were acquired for most samples. All these procedures were completed within 27 to 33 hours of BM aspiration for every patient. This time period was less than the recommended upper limit of cell sample storage for FCM (72 hours),24 and there was no difference in time to completion of FCM between LGw/oRS patients and controls.
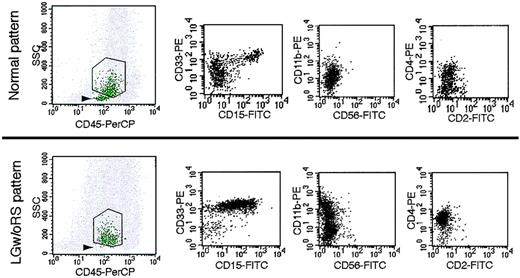
Data were analyzed with CellQuest software (BD). The investigators who performed FCM analyses were blinded to the patients' clinical and laboratory data, including the diagnoses, until all analyses were completed. Figure 1 shows the scheme of data analysis. On the forward scatter (FSC) versus side scatter (SSC) display, cells with relatively low SSC were gated (R1; Figure 1A). We plotted these gated cells on a CD45 versus CD34 display and gated CD34+ cells (R2; Figure 1B). Next, these CD34+ cells were plotted on a CD45 versus SSC display (green dots in Figure 1C), in which CD34+CD10+CD19+ B-cell precursors (stage I hematogones) formed an easily recognizable cluster that had the lowest SSC and relatively low CD45 expression among CD34+ cells (marked by the arrowhead in Figure 1C), as reported by others and us.19,25 CD34+ myeloblasts (CD34+ cells in R3; Figure 1C) showed more SSC and wider distribution of CD45 expression. We confirmed the reliability of the gating in every case (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Then we examined the following parameters: (1) expression of various antigens (CD2, CD4, CD10, CD11b, CD13, CD15, CD33, CD56, CD117, CD123, and CD133) on CD34+ myeloblasts as quantified by relative mean fluorescence intensity (RMFI, defined as the MFI of each antigen staining divided by the MFI of isotype-matched negative control staining) rather than percentage of antigen-positive cells because the former is more reproducible; (2) percentage of stage I hematogones in all CD34+ cells; and (3) percentage of CD34+ myeloblasts in all nucleated cells.
Statistical analyses
Differences between the 2 groups of data of continuous variables were analyzed with the Student t test. Differences in categorical variables were evaluated with the χ2 test. We established the RRs of flow cytometric parameters with data from controls in the first cohort. To establish the RRs, we used the mean ± 2 standard deviations (SD) or the receiver-operator characteristic (ROC) curve (see “Results”). To estimate the diagnostic power of the present FCM method, we calculated 95% confidence intervals for the binomial proportion according to the F distribution method.26
Outline of data analysis. (A) On the forward scatter (FSC) versus SSC display, cells in R1 were gated. (B) Gated cells in panel A were plotted on the CD45 versus CD34 display to gate CD34+ cells (R2). (C) Gated CD34+ cells were plotted on the CD45 versus SSC display (green dots) to identify stage I hematogones (CD34+CD10+CD19+ B-cell precursors [arrowhead]) and CD34+ myeloblasts (R3). Gray dots indicate other cells. The percentage of stage I hematogones in all CD34+ cells and the percentage of CD34+ myeloblasts in all nucleated cells was then determined. (D) Expression of various antigens on CD34+ myeloblasts gated in panel C was quantified by RMFI (MFI of each antigen staining divided by MFI of isotype-matched negative control staining).
Outline of data analysis. (A) On the forward scatter (FSC) versus SSC display, cells in R1 were gated. (B) Gated cells in panel A were plotted on the CD45 versus CD34 display to gate CD34+ cells (R2). (C) Gated CD34+ cells were plotted on the CD45 versus SSC display (green dots) to identify stage I hematogones (CD34+CD10+CD19+ B-cell precursors [arrowhead]) and CD34+ myeloblasts (R3). Gray dots indicate other cells. The percentage of stage I hematogones in all CD34+ cells and the percentage of CD34+ myeloblasts in all nucleated cells was then determined. (D) Expression of various antigens on CD34+ myeloblasts gated in panel C was quantified by RMFI (MFI of each antigen staining divided by MFI of isotype-matched negative control staining).
Results
Determining RRs with data from the first cohort
Representative patterns of FCM for controls and LGw/oRS patients are compared in Figure 2, and data from all controls and LGw/oRS patients in the first cohort are summarized in Table 2. In controls, stage I hematogones usually formed an easily detectable cluster (Figure 2, upper left), and they comprised 14.85% of all CD34+ cells (mean of 50 controls; Table 2). However, in the 12 LGw/oRS patients in this cohort, the stage I hematogone cluster often was undetectable or faint (Figure 2, lower left), and the hematogones comprised only 3.9% of all CD34+ cells (mean of 12 patients; Table 2). In the controls, as expected, CD34+ myeloblasts expressed CD13, CD33, and CD117 well (mean RMFI values of these antigens were 7.14-11.11; Table 2) but not antigens relating to myeloid cell maturation (CD11b and CD15)25 or lymphoid cells (CD2, CD4, CD10, and CD56) (mean RMFI values of these 6 antigens were 0.93-2.69; Table 2). In the LGw/oRS patients, CD34+ myeloblasts often aberrantly expressed CD4, CD11b, CD15, and CD56 and overexpressed CD13, CD33, CD117, and CD133 (examples for CD4, CD11b, CD15, and CD33 are shown in the lower panel of Figure 2).
Flow cytometric data from 50 controls and 12 LGw/oRS patients in the first cohort
. | Controls . | . | . | . | LGw/oRS patients . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FCM data . | Mean . | SD . | RR . | No. outside RR* . | Mean . | SD . | No outside RR* . | |||||
| Stage I hematogones in all CD34+ cells | 14.85 | 8.42 | > 4.50 | 4 | 3.90 | 2.94 | 8 | |||||
| CD34+ myeloblasts in all nucleated cells | 1.34 | 0.51 | < 2.36 | 0 | 1.76 | 1.34 | 2 | |||||
| Antigen expression on CD34+ myeloblasts | ||||||||||||
| Lymphoid antigens | ||||||||||||
| CD2 | 1.11 | 0.17 | < 1.45 | 1 | 1.00 | 0.10 | 0 | |||||
| CD4 | 1.86 | 0.47 | < 2.80 | 1 | 3.06 | 2.78 | 3 | |||||
| CD10 | 1.00 | 0.19 | < 1.39 | 2 | 0.83 | 0.09 | 0 | |||||
| CD56 | 0.93 | 0.13 | < 1.19 | 1 | 1.17 | 0.48 | 3 | |||||
| Myeloid antigens | ||||||||||||
| CD11b | 1.80 | 0.34 | < 2.49 | 1 | 2.25 | 1.19 | 3 | |||||
| CD13 | 11.11 | 4.49 | < 20.08 | 1 | 13.47 | 6.76 | 3 | |||||
| CD15 | 2.69 | 0.73 | < 4.14 | 1 | 5.42 | 4.60 | 6 | |||||
| CD33 | 7.14 | 3.62 | < 14.37 | 2 | 12.17 | 8.26 | 3 | |||||
| Immaturity-related antigens | ||||||||||||
| CD117 | 9.87 | 3.41 | < 16.70 | 3 | 13.89 | 9.53 | 4 | |||||
| CD123 | 2.12 | 1.00 | < 4.11 | 1 | 2.30 | 0.67 | 0 | |||||
| CD133 | 2.30 | 0.69 | < 3.68 | 2 | 2.25 | 1.30 | 3 | |||||
. | Controls . | . | . | . | LGw/oRS patients . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FCM data . | Mean . | SD . | RR . | No. outside RR* . | Mean . | SD . | No outside RR* . | |||||
| Stage I hematogones in all CD34+ cells | 14.85 | 8.42 | > 4.50 | 4 | 3.90 | 2.94 | 8 | |||||
| CD34+ myeloblasts in all nucleated cells | 1.34 | 0.51 | < 2.36 | 0 | 1.76 | 1.34 | 2 | |||||
| Antigen expression on CD34+ myeloblasts | ||||||||||||
| Lymphoid antigens | ||||||||||||
| CD2 | 1.11 | 0.17 | < 1.45 | 1 | 1.00 | 0.10 | 0 | |||||
| CD4 | 1.86 | 0.47 | < 2.80 | 1 | 3.06 | 2.78 | 3 | |||||
| CD10 | 1.00 | 0.19 | < 1.39 | 2 | 0.83 | 0.09 | 0 | |||||
| CD56 | 0.93 | 0.13 | < 1.19 | 1 | 1.17 | 0.48 | 3 | |||||
| Myeloid antigens | ||||||||||||
| CD11b | 1.80 | 0.34 | < 2.49 | 1 | 2.25 | 1.19 | 3 | |||||
| CD13 | 11.11 | 4.49 | < 20.08 | 1 | 13.47 | 6.76 | 3 | |||||
| CD15 | 2.69 | 0.73 | < 4.14 | 1 | 5.42 | 4.60 | 6 | |||||
| CD33 | 7.14 | 3.62 | < 14.37 | 2 | 12.17 | 8.26 | 3 | |||||
| Immaturity-related antigens | ||||||||||||
| CD117 | 9.87 | 3.41 | < 16.70 | 3 | 13.89 | 9.53 | 4 | |||||
| CD123 | 2.12 | 1.00 | < 4.11 | 1 | 2.30 | 0.67 | 0 | |||||
| CD133 | 2.30 | 0.69 | < 3.68 | 2 | 2.25 | 1.30 | 3 | |||||
Mean, SD, and RR are percentages (stage I hematogones in all CD34+ cells and CD34+ myeloblasts in all nucleated cells) or RMFI (other parameters). For controls, n = 50; for LGw/oRS patients, n = 12.
Number of patients or controls outside RR, out of all patients or controls analyzed.
Based on data from the 50 controls, we determined the RRs of 13 flow cytometric parameters (Table 2). In these controls, RMFI values of the examined antigens on CD34+ myeloblasts and percentages of CD34+ myeloblasts in all nucleated cells showed Gaussian distribution. As stated, in LGw/oRS patients in this cohort, data for these parameters often deviated upward, not downward, from those in controls. Therefore, we defined the RRs of these parameters as less than mean plus 2 SD of the data from the 50 controls. Because the data regarding the percentage of stage I hematogones in all CD34+ cells did not show a Gaussian distribution in controls, we used the ROC curve to determine the RR of the stage I hematogone percentage, which was greater than 4.5%.
Representative patterns of FCM of controls and LGw/oRS patients. Stage I hematogones usually formed an easily detectable cluster (arrowhead, top left panel) in controls but were undetectable or faint in LGw/oRS patients (arrowhead, bottom left panel). Abnormally high expression of CD15, CD33, CD11b, and CD4 was observed in LGw/oRS patients (3 bottom right panels) in comparison with the normal expression pattern of these antigens in a control (3 top right panels).
Representative patterns of FCM of controls and LGw/oRS patients. Stage I hematogones usually formed an easily detectable cluster (arrowhead, top left panel) in controls but were undetectable or faint in LGw/oRS patients (arrowhead, bottom left panel). Abnormally high expression of CD15, CD33, CD11b, and CD4 was observed in LGw/oRS patients (3 bottom right panels) in comparison with the normal expression pattern of these antigens in a control (3 top right panels).
Scoring system based on RRs
Table 2 shows the numbers of LGw/oRS patients and controls in the first cohort whose flow cytometric data were outside the RRs for each of the 13 flow cytometric parameters. Data on the expression of CD2, CD10, and CD123 on CD34+ myeloblasts were within the RRs in all 12 LGw/oRS patients. However, data on other parameters in these LGw/oRS patients were outside the RRs with various frequencies; the most frequent was stage I hematogones (8 of 12 [67%] patients) followed by CD15 (6 of 12 [50%] patients) and CD117 (4 of 12 [33%] patients). In most controls, however, data on these parameters were within the RRs. Then we performed FCM scoring, by which one point was given to each flow cytometric parameter except CD2, CD10, and CD123, listed in Table 2, if the data for each were outside the RR. For example, if the data for stage I hematogones, CD15, and CD117 were outside the RRs in a patient, the FCM score was 3.
Scores for controls and LGw/oRS patients in the first cohort are shown in Table 3 (left). Forty of the 50 (80%) controls had an FCM score of 0, and none had an FCM score of 3 or higher. Among LGw/oRS patients, 7 of 12 (58%) had an FCM score of 3 or higher. Therefore, using this scoring system, we detected LGw/oRS patients in the first cohort with 58% sensitivity and 100% specificity.
FCM scores of controls and LGw/oRS patients
. | First cohort . | . | Second cohort . | . | Both cohorts . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | Controls, no. . | LGw/oRS patients, no. . | Controls, no. . | LGw/oRS patients, no. . | Controls, no. . | LGw/oRS patients, no. . | |||
| Total | 50 | 12 | 40 | 15 | 90 | 27 | |||
| Score | |||||||||
| 0 | 40 | 1 | 31 | 2 | 71 | 3 | |||
| 1 | 4 | 1 | 9 | 1 | 13 | 2 | |||
| 2 | 6 | 3 | 0 | 3 | 6 | 6 | |||
| 3 | 0 | 2 | 0 | 4 | 0 | 6 | |||
| 4 | 0 | 1 | 0 | 3 | 0 | 4 | |||
| 5 | 0 | 3 | 0 | 1 | 0 | 4 | |||
| 6 | 0 | 1 | 0 | 0 | 0 | 1 | |||
| 7 | 0 | 0 | 0 | 1 | 0 | 1 | |||
| 3 or higher | 0 | 7 | 0 | 9 | 0 | 16 | |||
. | First cohort . | . | Second cohort . | . | Both cohorts . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | Controls, no. . | LGw/oRS patients, no. . | Controls, no. . | LGw/oRS patients, no. . | Controls, no. . | LGw/oRS patients, no. . | |||
| Total | 50 | 12 | 40 | 15 | 90 | 27 | |||
| Score | |||||||||
| 0 | 40 | 1 | 31 | 2 | 71 | 3 | |||
| 1 | 4 | 1 | 9 | 1 | 13 | 2 | |||
| 2 | 6 | 3 | 0 | 3 | 6 | 6 | |||
| 3 | 0 | 2 | 0 | 4 | 0 | 6 | |||
| 4 | 0 | 1 | 0 | 3 | 0 | 4 | |||
| 5 | 0 | 3 | 0 | 1 | 0 | 4 | |||
| 6 | 0 | 1 | 0 | 0 | 0 | 1 | |||
| 7 | 0 | 0 | 0 | 1 | 0 | 1 | |||
| 3 or higher | 0 | 7 | 0 | 9 | 0 | 16 | |||
* One point was given for each FCM parameter selected from Table 2 (ie, stage I hematogones, CD34+ myeloblasts, CD4, CD56, CD11b, CD13, CD15, CD33, CD117, and CD133) if the data on each parameter were outside the RR.
Prospective analysis of the second cohort using the scoring system
We then continued FCM analysis of the second cohort by applying the same scoring system and using the same RR values that had been determined in the first cohort. In the second cohort, we again observed that data on the expression of CD2, CD10, and CD123 on CD34+ myeloblasts were within the RRs in all LGw/oRS patients, whereas data on other parameters in LGw/oRS patients were outside the RRs with various frequencies (the most frequent was in stage I hematogones) (Table 4). Scoring in the second cohort revealed that most controls had FCM scores of 0 and no controls had FCM scores of 2 or higher. However, in LGw/oRS patients, 12 of 15 (80%) had FCM scores of 2 or higher and 9 of 15 (60%) had FCM scores of 3 or higher (Table 3, middle).
FCM data from 40 controls and 15 LGw/oRS patients in the second cohort (prospective analysis)
. | Controls . | . | . | LGw/oRS patients . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| FCM data . | Mean . | SD . | No. outside RR* . | Mean . | SD . | No. outside RR* . | ||||
| Hematogones in all CD34+ cells | 13.94 | 8.22 | 3 | 5.68 | 7.66 | 10 | ||||
| CD34+ myeloblasts in all nucleated cells | 1.11 | 0.54 | 1 | 2.07 | 1.63 | 4 | ||||
| Antigen expression on CD34+ myeloblasts | ||||||||||
| Lymphoid antigens | ||||||||||
| CD2 | 1.04 | 0.12 | 0 | 1.02 | 0.14 | 0 | ||||
| CD4 | 1.62 | 0.40 | 0 | 2.19 | 1.70 | 3 | ||||
| CD10 | 0.96 | 0.12 | 0 | 0.89 | 0.13 | 0 | ||||
| CD56 | 0.91 | 0.06 | 0 | 1.05 | 0.18 | 4 | ||||
| Myeloid antigens | ||||||||||
| CD11b | 1.61 | 0.33 | 0 | 1.46 | 0.55 | 1 | ||||
| CD13 | 10.10 | 4.14 | 2 | 20.98 | 15.93 | 6 | ||||
| CD15 | 2.33 | 0.62 | 0 | 3.06 | 1.98 | 3 | ||||
| CD33 | 5.34 | 1.46 | 0 | 9.38 | 5.56 | 2 | ||||
| Immaturity-related antigens | ||||||||||
| CD117 | 10.19 | 3.50 | 1 | 20.28 | 13.94 | 7 | ||||
| CD123 | 1.43 | 0.36 | 0 | 1.79 | 0.68 | 0 | ||||
| CD133 | 2.17 | 0.94 | 2 | 2.62 | 1.52 | 3 | ||||
. | Controls . | . | . | LGw/oRS patients . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| FCM data . | Mean . | SD . | No. outside RR* . | Mean . | SD . | No. outside RR* . | ||||
| Hematogones in all CD34+ cells | 13.94 | 8.22 | 3 | 5.68 | 7.66 | 10 | ||||
| CD34+ myeloblasts in all nucleated cells | 1.11 | 0.54 | 1 | 2.07 | 1.63 | 4 | ||||
| Antigen expression on CD34+ myeloblasts | ||||||||||
| Lymphoid antigens | ||||||||||
| CD2 | 1.04 | 0.12 | 0 | 1.02 | 0.14 | 0 | ||||
| CD4 | 1.62 | 0.40 | 0 | 2.19 | 1.70 | 3 | ||||
| CD10 | 0.96 | 0.12 | 0 | 0.89 | 0.13 | 0 | ||||
| CD56 | 0.91 | 0.06 | 0 | 1.05 | 0.18 | 4 | ||||
| Myeloid antigens | ||||||||||
| CD11b | 1.61 | 0.33 | 0 | 1.46 | 0.55 | 1 | ||||
| CD13 | 10.10 | 4.14 | 2 | 20.98 | 15.93 | 6 | ||||
| CD15 | 2.33 | 0.62 | 0 | 3.06 | 1.98 | 3 | ||||
| CD33 | 5.34 | 1.46 | 0 | 9.38 | 5.56 | 2 | ||||
| Immaturity-related antigens | ||||||||||
| CD117 | 10.19 | 3.50 | 1 | 20.28 | 13.94 | 7 | ||||
| CD123 | 1.43 | 0.36 | 0 | 1.79 | 0.68 | 0 | ||||
| CD133 | 2.17 | 0.94 | 2 | 2.62 | 1.52 | 3 | ||||
Mean, SD, and RR are percentages (hematogones in all CD34+ cells and CD34+ myeloblasts in all nucleated cells) or RMFI (other parameters). For the controls, n = 40; for LGw/oRS patients, n = 15.
Number of patients or controls outside RR, out of the patients or controls analyzed. RRs are the same as those shown in Table 2.
When data from both cohorts were combined, 16 of 27 LGw/oRS patients and 0 of 90 controls had FCM scores of 3 or higher (Table 3, right). Therefore, the sensitivity of LGw/oRS detection was 59% (95% confidence interval [95% CI], 39%-78%), and the specificity was 100% (95% CI, 96%-100%).
Degree of aberration of each FCM parameter
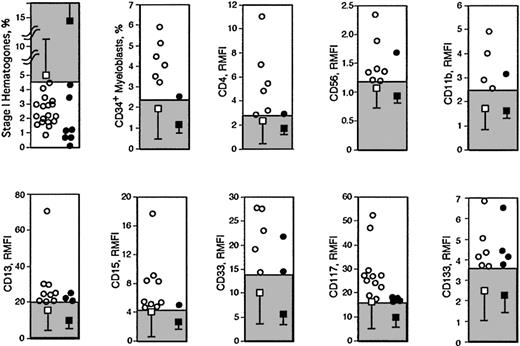
Data outside the RRs in controls and LGw/oRS patients in both cohorts for the parameters used for scoring are plotted in Figure 3. Although the percentage of stage I hematogones was frequently abnormally low in LGw/oRS patients, extremely low values were also observed in controls (Figure 3). In contrast, though abnormally high CD34+ myeloblast percentages were not frequent in LGw/oRS patients, CD34+ myeloblasts totaling 3% or greater were exclusively observed in LGw/oRS patients but not in controls (Figure 3). Similarly, markedly high RMFI values of CD4, CD15, and CD117 (more than approximately 4, 6, and 20, respectively) were exclusively observed in LGw/oRS patients (Figure 3). We speculate that the presence of such specific abnormalities in the samples examined may enhance the reliability of FCM diagnosis of LGw/oRS.
Age and sex do not affect LGw/oRS detection using the FCM score
Age and sex did not differ between controls and LGw/oRS patients in the first cohort (P = .81 and P = .52, respectively) (see the legend to Table 1 for age data). In the second cohort, sex did not differ but age differed significantly between controls and LGw/oRS patients (P = .35 and P = .002, respectively). However, in this cohort, age did not differ between controls with FCM scores of 1 and controls with FCM scores of 0 (57.0 ± 20.2 and 62.2 ± 13.4 years, respectively; P = .37). In addition, in 27 LGw/oRS patients, age and sex did not differ between patients with FCM scores of less than 3 and those with FCM scores of 3 or higher (P = .90 and P = .38, respectively).
Analysis of other clinical parameters in association with FCM scores in LGw/oRS patients
Results of FCM and clinical parameters in 27 LGw/oRS patients are summarized in Table 5. Detailed FCM data of these 27 patients are presented in Table S1. We examined whether the FCM score was associated with any other clinical parameter in LGw/oRS patients. The presence or absence of chromosomal aberrations was not associated with FCM score (P = .53; Table 6). However, when individual cytogenetic data were examined, patients with del(20q) or +8 (aberrations associated with specific prognoses [see Discussion]) often had FCM scores lower than 3, whereas all patients with other chromosomal aberrations had FCM scores of 3 or higher (P = .002; Table 6).
Characteristics and summary of diagnostic FCM in all LGw/oRS patients
. | . | . | . | . | FCM parameters . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Age, y/sex . | WHO classification . | IPSS . | Cytogenetics . | Stage I hematogones* . | CD34+ myeloblasts* . | Antigens on CD34+ myeloblasts for which expression (RMFI) was outside RRs . | FCM score . | ||
| 1 | 40/M | MDS-U | Low | 46,XY[20] | - | - | None | 0 | ||
| 2 | 70/M | MDS-U | Int-1 | 46,XY[20] | - | - | None | 0 | ||
| 3 | 86/M | RCMD | Int-1 | 46,XY[20] | - | - | None | 0 | ||
| 4 | 76/F | NA | Int-1 | 47,XX,+8[3]/46,XX[47] | + | - | None | 1 | ||
| 5 | 54/F | NA | Int-1 | 46,XX,t(6;7)(q21;p22),del(20)(q11)[9]/46,XX[11] | + | - | None | 1 | ||
| 6 | 37/M | RCMD | Int-1 | 46,XY[20] | - | - | CD56, CD13 | 2 | ||
| 7 | 77/M | RA | Int-1 | 45,X,-Y[4]/46,X,-Y,+8[16] | + | - | CD117 | 2 | ||
| 8 | 78/M | RA | Low | 46,XY,del(20)(q11)[2]/46,XY[18] | + | - | CD117 | 2 | ||
| 9 | 84/M | RCMD | Int-1 | 46,XY[20] | + | - | CD13 | 2 | ||
| 10 | 79/M | RA | Int-1 | 46,XY,del(20)(q11)[10]/47,XY,del(20)(q11), +22[1]/46,XY[9] | - | - | CD56, CD113 | 2 | ||
| 11 | 75/F | RCMD | Int-1 | 47,XX,+8[17]/47,XX,+8,del(13)(q12q14)[3] | + | - | CD4 | 2 | ||
| 12 | 75/F | RA | Int-1 | 46,XX,t(1;2)(q?41;q?31)[18]/46,XX[2] | + | - | CD56, CD15 | 3 | ||
| 13 | 82/M | MDS-U | Low | 46,XY[20] | - | - | CD11b, CD117, CD133 | 3 | ||
| 14 | 80/M | RCMD | Int-1 | 46,XY[20] | + | + | CD117 | 3 | ||
| 15 | 81/M | RCMD | Int-1 | 46,XY,del(20)(q11)[20] | + | - | CD56, CD15 | 3 | ||
| 16 | 74/F | RCMD | Int-1 | 46,XX[20] | - | - | CD56, CD15, CD33 | 3 | ||
| 17 | 78/M | RCMD | Int-1 | 46,XY,inv(6)(p23;q15)[20] | + | - | CD13, CD117 | 3 | ||
| 18 | 39/F | RA | Low | 46,XX[20] | + | - | CD56, CD15, CD33 | 4 | ||
| 19 | 69/M | RCMD | Int-2 | 46,XY,+1,der(1;7)(q10;p10)[6]/45,X,-Y[2]/46,XY[9] | - | - | CD4, CD13, CD117, CD133 | 4 | ||
| 20 | 85/F | RCMD | Int-1 | 46XX,add(18)(p11)[19]/46XX,i(17)(q10),add(18)(p11)[1] | + | + | CD13, CD117 | 4 | ||
| 21 | 75/M | RCMD | Int-1 | 47,XY,+13[2]/46,XY[18] | + | - | CD13, CD117, CD133 | 4 | ||
| 22 | 57/F | RCMD | NA | No metaphases | + | - | CD15, CD33, CD117, CD133 | 5 | ||
| 23 | 48/M | RA | Int-1 | 47,XX,+8[20] | + | - | CD4, CD13, CD15, CD133 | 5 | ||
| 24 | 73/F | RA | Low | 46,XX[20] | - | + | CD4, CD11b, CD15, CD33 | 5 | ||
| 25 | 70/M | RCMD | Int-1 | 46,XY,+1,der(1;7)(q10;p10)[9]/46,XY[11] | + | + | CD4, CD13, CD117 | 5 | ||
| 26 | 61/M | RCMD | Int-2 | 46,XY,+1,der(1;7)(q10;p10)[10]/46,X,del(Y)(q12),+1,der(1;7)(q10;p10)[5] | + | + | CD4, CD11b, CD13, CD15 | 6 | ||
| 27 | 64/F | RA | Int-2 | 45,XX,-7,inc[3]/46,XX[17] | + | + | CD56, CD11b, CD15, CD33, CD117 | 7 | ||
. | . | . | . | . | FCM parameters . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Age, y/sex . | WHO classification . | IPSS . | Cytogenetics . | Stage I hematogones* . | CD34+ myeloblasts* . | Antigens on CD34+ myeloblasts for which expression (RMFI) was outside RRs . | FCM score . | ||
| 1 | 40/M | MDS-U | Low | 46,XY[20] | - | - | None | 0 | ||
| 2 | 70/M | MDS-U | Int-1 | 46,XY[20] | - | - | None | 0 | ||
| 3 | 86/M | RCMD | Int-1 | 46,XY[20] | - | - | None | 0 | ||
| 4 | 76/F | NA | Int-1 | 47,XX,+8[3]/46,XX[47] | + | - | None | 1 | ||
| 5 | 54/F | NA | Int-1 | 46,XX,t(6;7)(q21;p22),del(20)(q11)[9]/46,XX[11] | + | - | None | 1 | ||
| 6 | 37/M | RCMD | Int-1 | 46,XY[20] | - | - | CD56, CD13 | 2 | ||
| 7 | 77/M | RA | Int-1 | 45,X,-Y[4]/46,X,-Y,+8[16] | + | - | CD117 | 2 | ||
| 8 | 78/M | RA | Low | 46,XY,del(20)(q11)[2]/46,XY[18] | + | - | CD117 | 2 | ||
| 9 | 84/M | RCMD | Int-1 | 46,XY[20] | + | - | CD13 | 2 | ||
| 10 | 79/M | RA | Int-1 | 46,XY,del(20)(q11)[10]/47,XY,del(20)(q11), +22[1]/46,XY[9] | - | - | CD56, CD113 | 2 | ||
| 11 | 75/F | RCMD | Int-1 | 47,XX,+8[17]/47,XX,+8,del(13)(q12q14)[3] | + | - | CD4 | 2 | ||
| 12 | 75/F | RA | Int-1 | 46,XX,t(1;2)(q?41;q?31)[18]/46,XX[2] | + | - | CD56, CD15 | 3 | ||
| 13 | 82/M | MDS-U | Low | 46,XY[20] | - | - | CD11b, CD117, CD133 | 3 | ||
| 14 | 80/M | RCMD | Int-1 | 46,XY[20] | + | + | CD117 | 3 | ||
| 15 | 81/M | RCMD | Int-1 | 46,XY,del(20)(q11)[20] | + | - | CD56, CD15 | 3 | ||
| 16 | 74/F | RCMD | Int-1 | 46,XX[20] | - | - | CD56, CD15, CD33 | 3 | ||
| 17 | 78/M | RCMD | Int-1 | 46,XY,inv(6)(p23;q15)[20] | + | - | CD13, CD117 | 3 | ||
| 18 | 39/F | RA | Low | 46,XX[20] | + | - | CD56, CD15, CD33 | 4 | ||
| 19 | 69/M | RCMD | Int-2 | 46,XY,+1,der(1;7)(q10;p10)[6]/45,X,-Y[2]/46,XY[9] | - | - | CD4, CD13, CD117, CD133 | 4 | ||
| 20 | 85/F | RCMD | Int-1 | 46XX,add(18)(p11)[19]/46XX,i(17)(q10),add(18)(p11)[1] | + | + | CD13, CD117 | 4 | ||
| 21 | 75/M | RCMD | Int-1 | 47,XY,+13[2]/46,XY[18] | + | - | CD13, CD117, CD133 | 4 | ||
| 22 | 57/F | RCMD | NA | No metaphases | + | - | CD15, CD33, CD117, CD133 | 5 | ||
| 23 | 48/M | RA | Int-1 | 47,XX,+8[20] | + | - | CD4, CD13, CD15, CD133 | 5 | ||
| 24 | 73/F | RA | Low | 46,XX[20] | - | + | CD4, CD11b, CD15, CD33 | 5 | ||
| 25 | 70/M | RCMD | Int-1 | 46,XY,+1,der(1;7)(q10;p10)[9]/46,XY[11] | + | + | CD4, CD13, CD117 | 5 | ||
| 26 | 61/M | RCMD | Int-2 | 46,XY,+1,der(1;7)(q10;p10)[10]/46,X,del(Y)(q12),+1,der(1;7)(q10;p10)[5] | + | + | CD4, CD11b, CD13, CD15 | 6 | ||
| 27 | 64/F | RA | Int-2 | 45,XX,-7,inc[3]/46,XX[17] | + | + | CD56, CD11b, CD15, CD33, CD117 | 7 | ||
NA indicates not applicable; Int, intermediate.
Plus signs indicate that percentage data were outside the RRs, and minus signs indicate that percentage data were within the RRs. Two NA cases (nos. 4 and 5) could not be classified because they had no morphologic abnormalities despite having chromosomal aberrations.
Association between FCM score and cytogenetics in LGw/oRS patients
. | Cytogenetics, no.* . | . | . | Patients with abnormal cytogenetics, no.† . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Abnormal . | Normal . | No metaphases . | +8 . | del(20q) . | der(1;7) . | Others . | |||||
| All patients | 16 | 10 | 1 | 4 | 4 | 3 | 5 | |||||
| FCM score | ||||||||||||
| 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | |||||
| 1 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | |||||
| 2 | 4 | 2 | 0 | 2 | 2 | 0 | 0 | |||||
| 3 | 3 | 3 | 0 | 0 | 1 | 0 | 2 | |||||
| 4 | 3 | 1 | 0 | 0 | 0 | 1 | 1 | |||||
| 5 | 2 | 1 | 1 | 1 | 0 | 1 | 0 | |||||
| 6 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | |||||
| 7 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |||||
| 3 or higher | 10 | 5 | 1 | 1 | 1 | 3 | 5 | |||||
. | Cytogenetics, no.* . | . | . | Patients with abnormal cytogenetics, no.† . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Abnormal . | Normal . | No metaphases . | +8 . | del(20q) . | der(1;7) . | Others . | |||||
| All patients | 16 | 10 | 1 | 4 | 4 | 3 | 5 | |||||
| FCM score | ||||||||||||
| 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | |||||
| 1 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | |||||
| 2 | 4 | 2 | 0 | 2 | 2 | 0 | 0 | |||||
| 3 | 3 | 3 | 0 | 0 | 1 | 0 | 2 | |||||
| 4 | 3 | 1 | 0 | 0 | 0 | 1 | 1 | |||||
| 5 | 2 | 1 | 1 | 1 | 0 | 1 | 0 | |||||
| 6 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | |||||
| 7 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |||||
| 3 or higher | 10 | 5 | 1 | 1 | 1 | 3 | 5 | |||||
Data are number of patients. Patients with +8, del(20q), and der(1;7) included those with additional cytogenetic aberrations.
Abnormal vs normal, P = .530 (proportions of patients with FCM scores of 3 or higher are compared).
+8 or del(20q) vs others, P = .002 (proportions of patients with FCM scores of 3 or higher are compared).
In general, most patients with RA diagnosed based on FAB criteria are subclassified into RA and RCMD in the WHO classification. MDS-U in the WHO classification represents a small population probably consisting of diverse patients.27 The clinical significance of this category remains unclear. In our LGw/oRS cohort, in addition to 3 MDS-U patients, 2 patients who had normal morphology but with chromosomal aberrations were included. Four of these 5 patients had FCM scores lower than 3 (0 or 1), and 15 of 22 patients with RA and RCMD according to the WHO classification had FCM scores of 3 or higher (P = .048).
Discussion
In our previous study,19 we prepared blast-enriched cell fractions using a novel density-centrifugation reagent designed for blast enrichment from 95 MDS patients, including 19 RA patients with diagnoses based on the FAB criteria, and then we performed immunophenotyping. MDS blasts were myeloblasts (CD13+, CD33+, or both). Seventy-nine percent of blasts (mean of the 19 RA patients) gated with the CD45 gating method expressed CD34. The cluster of immature B cells (hematogones) detected in normal samples treated according to the same centrifugation method was virtually absent in 15 of 17 RA patients examined. MDS blasts often exhibited aberrant antigen expression when compared with the data on normal myeloblasts reported by others. In the present study, we did not use the blast-enrichment method and focused instead on CD34+ cell characteristics primarily because the centrifugation method used, such as the blast-enrichment method, changed the cell composition of samples (eg, the percentage of immature B cells among total cells) and because data on CD34+ cells were more relevant to evaluate aberrant antigen expression than were data on blasts, which were gated on an SSC/CD45 cytogram and might have been contaminated by other cells. Furthermore, the present study used 4-color rather than 3-color FCM because the former required fewer cells (roughly half the number) to obtain the necessary data relating to multiple antigens. Using 4-color FCM, we were able to analyze the full set of data from all LGw/oRS patients examined. This is the first flow cytometric study to clarify the broad range of CD34+ cell–related abnormalities in low-grade MDS and to apply those abnormalities to the diagnosis of low-grade MDS. In addition, this study was objective in terms of incorporating the RR values obtained from control patients into data interpretation.
Data outside the RRs from both cohorts. Shaded areas are RRs. Data outside the RRs are shown in LGw/oRS patients (○) and controls (•). □ with bars indicates mean ± SD of all LGw/oRS patients; ▪ with bars, mean ± SD of all controls.
Data outside the RRs from both cohorts. Shaded areas are RRs. Data outside the RRs are shown in LGw/oRS patients (○) and controls (•). □ with bars indicates mean ± SD of all LGw/oRS patients; ▪ with bars, mean ± SD of all controls.
In the parameters related to CD34+ cells, a decrease in stage I hematogones was the most frequent LGw/oRS-related abnormality. This finding is not surprising because MDS clonal cells, which differentiate into myeloid cells but usually not into B lymphocytes,28-31 are predominant in the BM. Recently, Sternberg et al32 have reported the gene expression profile of CD34+ cells isolated from LGw/oRS patients. They found that decreased expression of B-cell lineage-affiliated genes was the most consistent finding in 26 LGw/oRS patients studied. They then used FCM to confirm a reduced percentage of stage I hematogones in the BM of LGw/oRS patients and proposed that this finding is a possible diagnostic biomarker for LGw/oRS diagnosis. Our results in this study showed that reduced stage I hematogones alone was not sufficient for the accurate diagnosis of LGw/oRS; a similar reduction was observed in 7 of 90 controls (2 NHL, 1 immune thrombocytopenic purpura in pregnancy, 1 anemia of chronic disease [pancreatic cancer], 1 pure white cell aplasia, 1 alcoholic cytopenia, 1 hypothermia-induced cytopenia).
By examining other flow cytometric parameters, we found that CD34+ cells from LGw/oRS patients often aberrantly expressed lymphoid antigens (CD4 and CD56) and antigens associated with myeloid cell maturation (CD11b, CD15)25 as well as overexpressed antigens present on normal CD34+ myeloblasts (CD13, CD33, CD117, CD133). It was also noted that in some LGw/oRS patients (patients 13, 22, 23, and 27; Table 5), CD34+ myeloblasts showed increased expression of both immature antigens (CD117, CD133, or both) and antigens associated with myeloid cell maturation (CD11b, CD15, or both). Therefore, these data formally confirmed that the antigen expression pathway is often deranged, which results in a variety of phenotypic aberrations, in CD34+ cells of LGw/oRS patients. A similar derangement has been well documented in blasts from patients with acute myeloid leukemia.17,18,33 We also observed that the percentage of CD34+ myeloblasts in LGw/oRS was higher than the RR in some patients and greater than 5% in 2 patients though blast percentages were lower than 5% in BM smears from all patients. Because of the fragility of MDS cells, we assumed that some BM cells (eg, some erythroblasts and mature myeloid cells) might have been broken during erythrocyte lysing, antibody staining, and washing before FCM, possibly artificially increasing the percentage of CD34+ myeloblasts in our samples.
At present, if morphologic diagnosis of LGw/oRS is uncertain, only a chromosomal abnormality can confirm LGw/oRS.34 If such patients have normal karyotype, it is recommended that they undergo PB and BM examinations at appropriate intervals to confirm the diagnosis.34 In this study, we used phenotypic aberrations of CD34+ cells in the diagnosis of LGw/oRS. The objectivity and reproducibility of our method are sufficiently high that other laboratories use our strategy. When we defined an FCM score of 3 or higher as indicating LGw/oRS, we were able to diagnose LGw/oRS in 16 of 27 patients, irrespective of the presence or absence of chromosomal aberrations. These findings indicate that our method, similar to chromosomal analysis, can confirm the diagnosis of LGw/oRS. By adding our method to chromosomal analysis, we were able to diagnose LGw/oRS in more patients; in our cohort, only 5 of 27 LGw/oRS patients (patients 1-3, 6, 9; Table 5) had neither chromosomal aberrations nor FCM scores of 3 or higher. We also speculate that if, in addition to the present FCM parameters, other FCM parameters, including data from gated mature cells, are included in the analysis, the sensitivity of LGw/oRS detection might increase.
There are reports of rare patients with cytopenia (or macrocytosis) and abnormal marrow cytogenetics whose hematopoietic cells are of normal morphology including normal maturation and normal cytochemistry.35,36 Therefore, MDS cannot be diagnosed based on FAB and WHO classifications. In such patients, del(20q) is a common cytogenetic aberration, and trisomy 8 is not uncommon.36 Patients 4 and 5 in this study, who had trisomy 8 and del(20q), respectively, but normal morphology, were in this category. MDS patients with del(20q) were identified in the IPSS study as having a favorable prognosis,22 and trisomy 8 MDS patients have recently shown good response to immunosuppressive therapies.37 In this study, among LGw/oRS patients with chromosomal aberrations, patients with trisomy 8 or del20(q) had low FCM scores. Although the biologic background explaining this association must be studied further, our data may support the concept that patients with these karyotypes show a specific biologic behavior among the heterogeneous MDS population.
In conclusion, we have clarified the broad range of CD34+ cell–related abnormalities and used them to develop a well-controlled, objective FCM approach to the diagnosis of LGw/oRS. We believe that this objective analysis has diagnostic value and provides a new tool that deepens our understanding of the biology of MDS.
Prepublished online as Blood First Edition Paper, March 30, 2006; DOI 10.1182/blood-2005-12-4916.
Supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (no. 17591015).
K.O. designed the research, performed morphologic analysis, collected clinical data, analyzed the data, and wrote the manuscript. Y.K. performed flow cytometric analysis. Y.K., C.S., and A.H. analyzed the flow cytometric data. H.T. collected clinical data. K.D. performed morphologic analysis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Professor Raul Braylan for advising on the study design and critically reviewing the data and manuscript.

![Figure 1. Outline of data analysis. (A) On the forward scatter (FSC) versus SSC display, cells in R1 were gated. (B) Gated cells in panel A were plotted on the CD45 versus CD34 display to gate CD34+ cells (R2). (C) Gated CD34+ cells were plotted on the CD45 versus SSC display (green dots) to identify stage I hematogones (CD34+CD10+CD19+ B-cell precursors [arrowhead]) and CD34+ myeloblasts (R3). Gray dots indicate other cells. The percentage of stage I hematogones in all CD34+ cells and the percentage of CD34+ myeloblasts in all nucleated cells was then determined. (D) Expression of various antigens on CD34+ myeloblasts gated in panel C was quantified by RMFI (MFI of each antigen staining divided by MFI of isotype-matched negative control staining).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/3/10.1182_blood-2005-12-4916/4/m_zh80150699260001.jpeg?Expires=1769156387&Signature=lr63quyjxhAqh36ajMHHWi~js4~V2wjvZoVf-T1pKSoI-cH1w3tqBfdE6b86QxWxQze8I0LzFb4yX4KWHrraa5PmpUcVBcGTPUX~STDQO0aGKSkeEfyAzmqterc2e-Dgsvk8UhvUFAiLPaxJtVNimRROOWy6X0kD7v7t1-Y5r0vNLnMokqCv7eS6r8JjfYtlbnBIxnb5seZML-lpbVrNfTJ2TG8tabDJM-Rufl~Fi~Z4pfDnevj2xSftd2zX3gzOiWL9aQOZOqEiuAEDIy~ziYJ-JMLtdL6wdBjDs-7JqDAc4TUK0JsLNVi1hStlH8Si51ZArhOZ6vGPQVyc7QMvtQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal