The immune system of patients with severe combined immunodeficiency (SCID) reconstitutes to a large extent during the first years after hematopoietic stem cell transplantation (HSCT). It was suggested, however, that accelerated loss of thymus output may cause impaired immune function at the long term. To address this issue, we studied patients with SCID who underwent allogeneic HSCT 5 to 32 years earlier and identified early determinants of long-term T-cell reconstitution. A variety of immune parameters were analyzed both early (1-4 years) and late (5-32 years) after HSCT. Late after HSCT, a clear distinction could be made between a group of 8 patients with impaired T-cell reconstitution and 11 patients with good immune reconstitution. Importantly, in patients with decreased long-term T-cell reconstitution, T-cell recovery was already poor early after HSCT, demonstrating that long-term immune failure was not caused by accelerated loss of thymus output or long-term graft failure, but resulted from poor early grafting. The number of T-cell receptor excision circles (TRECs) early after HSCT was most predictive for long-term T-cell reconstitution. Frequent monitoring of T-cell immunity and TREC numbers early after HSCT may thus serve to timely identify patients who will fail to reconstitute properly and who may need additional treatment.
Introduction
Severe combined immunodeficiency (SCID) is a group of inherited disorders characterized by severely impaired cellular and humoral immunity, which causes an increased risk of persistent opportunistic infections and is generally fatal at very early age if untreated.1,2 Since 1968 patients with SCID have been successfully treated using allogeneic hematopoietic stem cell transplantation (HSCT).3,4 Ideally, stem cells are obtained from HLA-identical siblings, giving a current 3-year survival rate of approximately 85%.5 HLA-haploidentical parental donors are used with increasing success.5,6 Several factors, including the lack or proper prevention of graft-versus-host disease (GvHD),7 types of SCID in which B cells are present,8 and transplantation in the neonatal period,9 are associated with better outcome after HSCT. Moreover, T-cell reconstitution in the first 6 months after HSCT was found to be strongly associated with survival.7
Generally, T-cell immunity restores within about a year after HSCT, whereas B-cell immunity restores more slowly.7,10-12 It remains unclear, however, whether the various T-cell subsets in patients with SCID normalize similarly within the first years after HSCT when compared with healthy age-matched control subjects. Reconstitution of naive T cells in patients with SCID was found to coincide with normalization of the size of the thymus.9,13 Because of thymus-dependent T-cell generation, not only naive T-cell counts but also T-cell diversity increase during the first year after HSCT.14
Despite considerable T-cell reconstitution during the first years after HSCT in most patients with SCID, it has been suggested that T-cell immunity may be impaired later in life because of long-term graft failure or accelerated loss of thymus output.14,15 Until now, only a few studies of long-term T-cell reconstitution after HSCT for SCID have been performed, and longitudinal data are scarce. Buckley et al6 reported normal T-cell responses to mitogens and normal total T-cell counts in a cross-sectional study of 89 patients with SCID who had been treated with HLA-matched or haploidentical HSCT up to 17 years (median, 5.6 years) earlier. However, Patel et al15 found that the average number of naive T cells and the level of T-cell proliferation in response to mitogens decreased with time after transplantation in a group of 83 patients with SCID who were investigated up to 14 years after transplantation. To study whether these changes could be due to an accelerated loss of thymus output, T-cell receptor excision circles (TRECs) were measured. TRECs are extrachromosomal DNA circles, which are formed during T-cell receptor rearrangement in the thymus and are not replicated on T-cell division. TRECs were investigated in 51 patients with SCID of whom 16 reached a follow-up of at least 5 years after HSCT and 3 were evaluated longitudinally. Because the average TREC content of peripheral blood mononuclear cells (PBMCs) in patients with SCID after HSCT declined faster than the typical age-related TREC decline in healthy control subjects, it was proposed that the thymus of patients with SCID may not be capable of sustaining sufficient output, possibly causing long-term infectious problems.15
Recent clinical follow-up of long-term survivors after HSCT for SCID in our transplantation unit has suggested an increased incidence of infections and autoimmune disorders late after HSCT. We, therefore, set out to study T-cell immunity longitudinally in all patients with SCID who received a transplant in our clinic between 5 and 32 years earlier, to establish whether T-cell immunity or thymus-dependent T-cell lymphopoiesis showed an accelerated decrease with age. By analyzing blood samples that were collected early (between 1 and 4 years) and late (between 5 and 32 years) after HSCT, and by comparing the data with age-matched healthy control values, we were able to define early determinants of long-term T-cell reconstitution.
Patients and methods
Cohort of patients with SCID
Between 1968 and 1997, 35 patients with SCID were treated with allogeneic HSCT at the Pediatric Transplant Unit of Leiden University Medical Center (LUMC); 21 of them were alive 5 years after HSCT. Nineteen of these 21 patients and their parents could be contacted, and all were willing to participate in a study to evaluate their current state of acquired immunity. The cohort is heterogeneous with respect to the type of SCID, the graft donor, the manipulation of the bone marrow (BM) graft, conditioning, the occurrence and grade of GvHD, and GvHD prophylaxis (Table 1). Three patients showed a failure of engraftment and received another transplant 4 to 6 months after the first attempt. The date of second HSCT was considered as the starting point of follow-up in these cases. Total follow-up ranged from 5 to 32 years (median, 12 years) after HSCT. Informed consent was obtained from all participants or their parents. The review board for medical ethics of the LUMC approved the study.
Patient characteristics
. | . | . | . | . | . | . | . | . | . | . | . | . | FU† . | . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup and UPN . | Sex . | SCID phenotype* . | SCID genotype* . | Age at HSCT, mo . | Donor . | TCD graft . | Conditioning . | GvHD prev . | aGvHD grade . | cGvHD grade . | Clinical status at late FU . | Infections at late FU . | Early . | Late . | |
| Good reconstitution | |||||||||||||||
| 17‡ | F | T-B+NK+/- | ? (AR) | 33 | IRD | Alb. gradient | — | — | — | — | — | Warts | 25.5 | ||
| 50‡ | F | T-B+NK- | ? (AR) | 18 | IRD | E-rosetting | TBI/CY | MTX | — | — | — | — | 1.5 | 18.3 | |
| 56 | F | T-B+NK- | ? (AR) | 9 | ORD | E-rosetting | CY | — | 2 | Extensive | Vasculitis | — | 1.5 | 17.4 | |
| 59 | M | T-B+NK+ | ? | 9 | ORD | E-rosetting | — | — | 2 | Extensive | — | — | 2.9 | 17.9 | |
| 96 | F | T-B-NK+/- | ADA | 8 | ORD | E-rosetting | BU/CY | CsA | 1 | — | Spastic dipl/retard | — | 1.0 | 15.1 | |
| 112 | M | T-B+NK- | γc (XL) | 5 | ORD | E-rosetting | BU/CY | CsA | 1 | Limited | — | — | 2.5 | 13.8 | |
| 199 | M | T-B+NK- | γc (XL) | 10 | ORD | E-rosetting | BU/CY | MTX | — | — | — | URTI/ENT | 1.6 | 8.5 | |
| 220 | F | T-B+NK- | ? (AR) | 18 | MUD | E-rosetting | BU/CY | MTX | — | — | — | — | 1.0 | 8.3 | |
| 221 | F | T-B+NK- | ? (AR) | 18 | MUD | E-rosetting | BU/CY | MTX | — | Limited | — | — | 1.0 | 8.3 | |
| 228 | M | T-B+NK- | γc (XL) | 7 | ORD | E-rosetting | BU/CY | CsA | — | — | — | — | 1.3 | 7.7 | |
| 304 | M | T-B+NK- | γc (XL) | 9 | ORD | E-rosetting | BU/CY | CsA/MTX | 2 | Limited | — | — | 1.7 | 6.1 | |
| Poor reconstitution | |||||||||||||||
| 2 | M | T-B+NK- | γc (XL) | 5 | IRD | Alb. gradient | — | — | — | — | Lymphedema | Warts/pneum | 3.9 | 32.2 | |
| 53‡ | M | T-B+NK+ | IL-7Rα | 12 | ORD | E-rosetting | CY | CsA | — | — | — | Warts | 1.6 | 16.9 | |
| 108 | M | T-B+NK+ | ? (AR) | 10 | ORD | E-rosetting | BU/CY | CsA | — | — | — | Resp tract | 1.0 | 13.8 | |
| 131 | M | T-B-NK+/- | ADA | 1 | IRD | No | BU/CY | CsA | — | — | — | — | 1.7 | 11.9 | |
| 168 | F | T-B-NK+ | Artemis | 8 | ORD | E-rosetting | BU/CY | CsA | — | — | Spastic dipl | — | 3.5 | 10.3 | |
| 183 | F | T-B-NK+ | RAG1-2 | 4 | ORD | E-rosetting | BU/CY | CsA | — | — | — | — | 1.0 | 6.3 | |
| 242 | M | T-B-NK+/- | ADA | 1 | IRD | No | — | CsA | — | — | Learning problems | — | 1.4 | 7.2 | |
| 306 | M | T-B-NK+ | RAG1-2 | 2 | IRD | No | — | — | 2 | Limited | — | — | 1.3 | 5.0 | |
. | . | . | . | . | . | . | . | . | . | . | . | . | FU† . | . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup and UPN . | Sex . | SCID phenotype* . | SCID genotype* . | Age at HSCT, mo . | Donor . | TCD graft . | Conditioning . | GvHD prev . | aGvHD grade . | cGvHD grade . | Clinical status at late FU . | Infections at late FU . | Early . | Late . | |
| Good reconstitution | |||||||||||||||
| 17‡ | F | T-B+NK+/- | ? (AR) | 33 | IRD | Alb. gradient | — | — | — | — | — | Warts | 25.5 | ||
| 50‡ | F | T-B+NK- | ? (AR) | 18 | IRD | E-rosetting | TBI/CY | MTX | — | — | — | — | 1.5 | 18.3 | |
| 56 | F | T-B+NK- | ? (AR) | 9 | ORD | E-rosetting | CY | — | 2 | Extensive | Vasculitis | — | 1.5 | 17.4 | |
| 59 | M | T-B+NK+ | ? | 9 | ORD | E-rosetting | — | — | 2 | Extensive | — | — | 2.9 | 17.9 | |
| 96 | F | T-B-NK+/- | ADA | 8 | ORD | E-rosetting | BU/CY | CsA | 1 | — | Spastic dipl/retard | — | 1.0 | 15.1 | |
| 112 | M | T-B+NK- | γc (XL) | 5 | ORD | E-rosetting | BU/CY | CsA | 1 | Limited | — | — | 2.5 | 13.8 | |
| 199 | M | T-B+NK- | γc (XL) | 10 | ORD | E-rosetting | BU/CY | MTX | — | — | — | URTI/ENT | 1.6 | 8.5 | |
| 220 | F | T-B+NK- | ? (AR) | 18 | MUD | E-rosetting | BU/CY | MTX | — | — | — | — | 1.0 | 8.3 | |
| 221 | F | T-B+NK- | ? (AR) | 18 | MUD | E-rosetting | BU/CY | MTX | — | Limited | — | — | 1.0 | 8.3 | |
| 228 | M | T-B+NK- | γc (XL) | 7 | ORD | E-rosetting | BU/CY | CsA | — | — | — | — | 1.3 | 7.7 | |
| 304 | M | T-B+NK- | γc (XL) | 9 | ORD | E-rosetting | BU/CY | CsA/MTX | 2 | Limited | — | — | 1.7 | 6.1 | |
| Poor reconstitution | |||||||||||||||
| 2 | M | T-B+NK- | γc (XL) | 5 | IRD | Alb. gradient | — | — | — | — | Lymphedema | Warts/pneum | 3.9 | 32.2 | |
| 53‡ | M | T-B+NK+ | IL-7Rα | 12 | ORD | E-rosetting | CY | CsA | — | — | — | Warts | 1.6 | 16.9 | |
| 108 | M | T-B+NK+ | ? (AR) | 10 | ORD | E-rosetting | BU/CY | CsA | — | — | — | Resp tract | 1.0 | 13.8 | |
| 131 | M | T-B-NK+/- | ADA | 1 | IRD | No | BU/CY | CsA | — | — | — | — | 1.7 | 11.9 | |
| 168 | F | T-B-NK+ | Artemis | 8 | ORD | E-rosetting | BU/CY | CsA | — | — | Spastic dipl | — | 3.5 | 10.3 | |
| 183 | F | T-B-NK+ | RAG1-2 | 4 | ORD | E-rosetting | BU/CY | CsA | — | — | — | — | 1.0 | 6.3 | |
| 242 | M | T-B-NK+/- | ADA | 1 | IRD | No | — | CsA | — | — | Learning problems | — | 1.4 | 7.2 | |
| 306 | M | T-B-NK+ | RAG1-2 | 2 | IRD | No | — | — | 2 | Limited | — | — | 1.3 | 5.0 | |
UPN indicates unique personal number; FU, follow-up; SCID genotype ?, unknown; AR, autosomal recessive; IRD, HLA-identical family donor; Alb, albumin; E-rosetting, rosetting of T cells to sheep erythrocytes; TBI, total body irradiation; CY, cyclophosphamide; MTX, methotrexate; ORD, haploidentical family donor; ADA, adenosine deaminase deficiency; BU, busulphan; CsA, cyclosporin A; spastic dipl, spastic diplegia; retard, retardation; γc, common γ chain deficiency; XL, X-chromosome linked; URTI, upper respiratory tract infections; ENT, ear, nose, and throat infections; MUD, matched unrelated donor; pneum, pneumonia; IL-7Rα, IL-7 receptor α chain deficiency; resp tract, respiratory tract; Artemis, DNA-repair defect resulting from mutations in the Artemis gene; RAG1-2, recombinase-activating gene (RAG1 or RAG2) deficiency. — indicates no complaints with respect to clinical status; no with respect to conditioning, GVHD prevention, and infections; and absent with respect to aGVHD and cGVHD.
Data in parentheses indicate the way of inheritance.
Early and late are given in years after HSCT.
Patients who received transplants twice.
Samples
PBMCs from patients with SCID and their donors were analyzed at 2 time points: a late time point during follow-up, between 5 and 32 years (median, 12 years) after HSCT (late follow-up [FU]) and an early time point between 1 and 4 years (median, 1.5 years) after HSCT (early FU). As controls, blood samples were taken from healthy Dutch adults (n = 10), children who were visiting the Academic Medical Center outpatient clinic for various conditions that were not related to immunopathologic or infectious diseases (n = 26),16 or HSC donors at the Pediatric Transplant Unit of the LUMC (n = 137). Viable PBMCs were stored in liquid nitrogen until analysis.
Peripheral blood lymphocyte (sub)populations and T-cell proliferation
Naive (CD45RA+) and memory (CD45RA–) T-cell counts within the CD3+CD4+ and CD3+CD8+ T-cell subsets, as well as CD19+/CD20+ B-cell counts and CD3–CD16/56+ natural killer (NK) cell counts were determined by flow cytometry as described previously.17 T-cell proliferation rates were measured ex vivo by flow cytometric analysis of Ki-67 nuclear antigen expression in naive and total CD4+ and CD8+ T cells, as described previously.18 Proliferative responses of PBMCs to the T-cell mitogen phytohemagglutinin (PHA; 5 μg/mL) were measured by 3H-thymidine incorporation. 3H-thymidine was added to the cultures after 3 days of stimulation, and its incorporation was analyzed 16 hours later. Chimerism of the different cell lineages was determined by XY-fluorescence in situ hybridization (FISH) or by polymerase chain reaction (PCR) amplification of short tandem repeats as previously described.19,20
TRECs and telomere lengths
DNA was purified from CD4+ or CD8+ T cells using the QIAamp Blood Kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. Signal joint TREC content of these fractions was quantified using real-time PCR as described previously.21 TREC content was expressed as the number of TREC copies per CD4+ or CD8+ T cell, assuming that 1 μg DNA represents 150 000 T cells. Telomere length was determined in naive (CD45RA+) and memory (CD45RA–) CD4+ T cells by a combination of immunostaining and flow-FISH analysis.22
Statistical analysis
Immune parameter differences between the 2 groups were analyzed using the Mann-Whitney U test. The proportion of the different SCID phenotypes, conditioning, and levels of chimerism in the 2 groups were compared using the Student t test, using pooled variances if there was no significant difference between the variances of the 2 groups (based on Levene test for equality of variances). P values less than .05 were considered significant.
Results
T-cell reconstitution long-term after HSCT
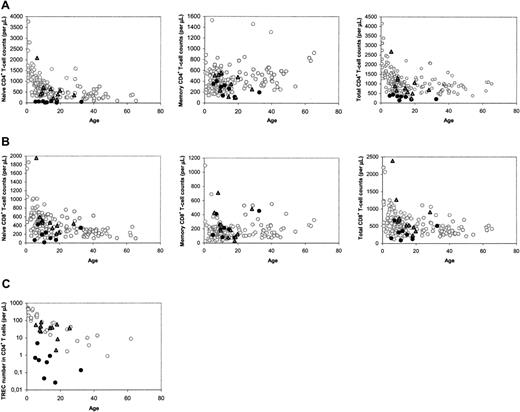
The median number of naive CD4+ T cells in patients with SCID long term (range, 5-32 years; median, 12 years) after HSCT was found to be lower than that of age-matched healthy control subjects, as was reported previously.15 However, close analysis of the data revealed a clear distinction between a group of 11 patients with SCID with normal and a group of 8 patients with decreased naive CD4+ T-cell counts at late follow-up (Figure 1A). In most cases, decreased naive CD4+ T-cell counts were accompanied by low total CD4+ T-cell counts (Figure 1A), and relatively low naive and total CD8+ T-cell counts (Figure 1B). At late follow-up, the median telomere length in naive (P = .001) and memory (P = .002) CD4+ T cells, the number of CD19+/CD20+ B cells (P = .001), and the in vitro response of PBMCs to the T-cell mitogen PHA (P = .045) were also found to be significantly higher in patients with good naive CD4+ T-cell reconstitution than with poor naive CD4+ T-cell reconstitution. The numbers of CD3–CD16/56+ NK cells in both groups were not statistically different (P = .16).
To analyze whether the distinction between the 2 groups of patients could be related to thymus output, we measured TREC contents in CD4+ and CD8+ T cells. At late follow-up, only individuals with poor T-cell reconstitution had reduced CD4+ TREC contents compared with age-matched healthy control subjects (data not shown). Differences in TREC contents may reflect differences in thymus output but may also be due to TREC dilution by peripheral T-cell proliferation.21 When the level of T-cell proliferation was analyzed by measuring Ki-67 expression in naive and total CD4+ T cells at late follow-up, we found no significant differences between patients and healthy control subjects, but patients with poor T-cell reconstitution had significantly higher levels of Ki-67 expression than individuals with good T-cell reconstitution (P = .006). Because increased T-cell proliferation rates could thus be the cause of lower TREC contents in individuals with poor long-term T-cell reconstitution, we also analyzed the total numbers of TRECs in CD4+ and CD8+ T cells per microliter of blood, which are not influenced by T-cell proliferation, as a more direct measure of thymus output. Again, a clear distinction between the 2 groups was observed: CD4+ TREC numbers were decreased in patients with impaired T-cell reconstitution at late follow-up (P < .001), whereas they were comparable to healthy age-matched control subjects in individuals with good long-term T-cell reconstitution (P = .495; Figure 1C). CD8+ TREC numbers were also significantly (P < .001) higher in patients with good long-term immune reconstitution than with poor long-term immune reconstitution (data not shown). This suggests that the number of thymus emigrants in patients with poor long-term T-cell reconstitution was reduced.
Immune parameters at late follow-up, between 5 and 32 years after HSCT. (A) CD4+ T-cell counts in the different subsets. (B) CD8+ T-cell counts in the different subsets. (C) CD4+ TREC numbers. ○ denotes healthy control subjects; •, patients with SCID at long-term follow-up with poor long-term reconstitution of the naive CD4+ T-cell population;  and, patients with SCID at long-term follow-up with good long-term reconstitution of the naive CD4+ T-cell population.
and, patients with SCID at long-term follow-up with good long-term reconstitution of the naive CD4+ T-cell population.
Immune parameters at late follow-up, between 5 and 32 years after HSCT. (A) CD4+ T-cell counts in the different subsets. (B) CD8+ T-cell counts in the different subsets. (C) CD4+ TREC numbers. ○ denotes healthy control subjects; •, patients with SCID at long-term follow-up with poor long-term reconstitution of the naive CD4+ T-cell population;  and, patients with SCID at long-term follow-up with good long-term reconstitution of the naive CD4+ T-cell population.
and, patients with SCID at long-term follow-up with good long-term reconstitution of the naive CD4+ T-cell population.
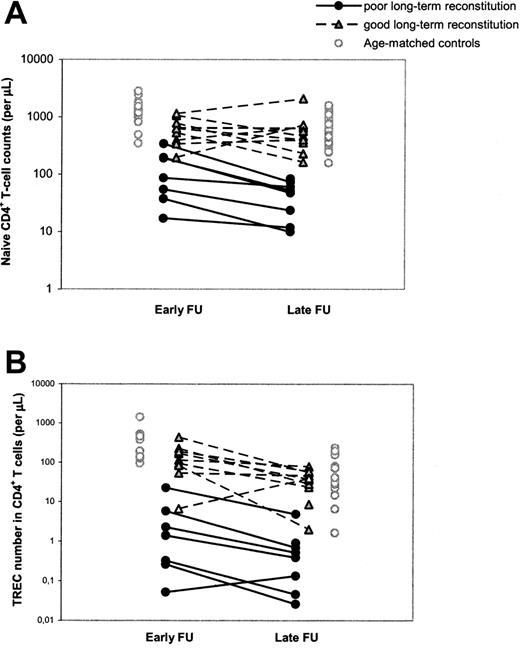
Differences in T-cell reconstitution are already evident early after HSCT
To study the possibility that poor T-cell reconstitution at the long term was due to an accelerated decline in thymus output or to long-term graft failure, as was previously suggested,14,15 we performed a longitudinal, retrospective analysis of T-cell immunity and thymus output in our cohort of patients with SCID by also investigating a time point early (range, 1-4 years; median, 1.5 years) after HSCT. It appeared that individuals with poor T-cell reconstitution at late follow-up already had significantly (P = .002) lower naive CD4+ T-cell counts at early follow-up compared with patients with good long-term T-cell reconstitution (Figure 2A; Table 2). In analogy, CD4+ TREC numbers per microliter of blood in patients with poor long-term T-cell reconstitution were already significantly lower (P = .002) early after HSCT compared with patients with good long-term T-cell reconstitution (Figure 2B; Table 2). The median period between HSCT and the time point of early follow-up was 1.5 years for both groups of patients and could thus not explain the differences in short-term T-cell reconstitution between the groups. Taken together, these data suggest that poor long-term T-cell reconstitution was neither caused by an accelerated loss of thymus output nor by long-term graft failure. In 11 of 19 patients with SCID, long-term T-cell reconstitution and thymus output after HSCT did not differ from healthy age-matched control subjects, whereas in the other group of patients with poor T-cell reconstitution and decreased thymus output at late follow-up, both impairments were already evident early after HSCT.
Early prediction of long-term T-cell reconstitution after HSCT for SCID
Parameter . | Control subjects . | SCID cohort . | P* . | Poor reconstitution . | Good reconstitution . | P† . |
|---|---|---|---|---|---|---|
| Counts of lymphocytes, per μL | ||||||
| Naive CD4+ T cells | 1432 | 341 | < .001‡ | 86 | 630 | .002‡ |
| Memory CD4+ T cells | 303 | 222 | .016‡ | 253 | 185 | .711 |
| Total CD4+ T cells | 1662 | 789 | .001‡ | 451 | 1254 | .003‡ |
| Naive CD8+ T cells | 603 | 300 | .041‡ | 146 | 323 | .153 |
| Memory CD8+ T cells | 150 | 75 | .246 | 69 | 80 | .672 |
| Total CD8+ T cells | 697 | 552 | .412 | 419 | 640 | .328 |
| Total CD19+/CD20+ B cells | 677 | 381 | .059 | 70 | 649 | .005‡ |
| Total CD3-CD16/56+ NK cells | 193 | 127 | .014‡ | 119 | 129 | .625 |
| Ki-67 expression, % | ||||||
| Total CD4+ T cells | 3.0 | 6.1 | .07 | 6.4 | 5.8 | .25 |
| Naive CD4+ T cells | 1.1 | 2.6 | .24 | 3.2 | 2.0 | .27 |
| TRECs | ||||||
| Content in CD4+ T cells, no. per cell | 0.20 | 0.03 | .006‡ | 0.007 | 0.11 | .005‡ |
| Content in CD8+ T cells, no. per cell | 0.10 | 0.04 | .082 | 0.002 | 0.10 | .013‡ |
| No. in CD4+ T cells/μL blood | 410 | 23 | .001‡ | 1.4 | 105 | .002‡ |
| No. in CD8+ T cells/μL blood | 101 | 16 | .039‡ | 0.2 | 51 | .005‡ |
| Telomere length, relative to donor | ||||||
| Naive CD4+ T cells | ND | NA | NA | 1.3 | 1.6 | .079 |
| Memory CD4+ T cells | ND | NA | NA | 1.1 | 1.3 | .240 |
| Other parameters | ||||||
| Age of patient at HSCT, y | NA | 0.7 | 0.4 | 0.7 | .020‡ | |
| Age of donor at HSCT, y | NA | 24.2 | 15.9 | 27.9 | .457 | |
| SCID phenotype, no. | ||||||
| NK- | NA | 9 | 1 | 8 | .007‡ | |
| B- | NA | 6 | 5 | 1 | .025‡ | |
| B cells predominantly of donor origin, no. | NA | 11 | 4 | 7 | .578 | |
| NK cells predominantly of donor origin, no. | NA | 11 | 2 | 9 | .023‡ | |
| Monocytes predominantly of donor origin, no. | NA | 5 | 0 | 5 | .016‡ | |
| Myeloablative conditioning, BU/CY, no. | NA | 11 | 4 | 7 | .578 |
Parameter . | Control subjects . | SCID cohort . | P* . | Poor reconstitution . | Good reconstitution . | P† . |
|---|---|---|---|---|---|---|
| Counts of lymphocytes, per μL | ||||||
| Naive CD4+ T cells | 1432 | 341 | < .001‡ | 86 | 630 | .002‡ |
| Memory CD4+ T cells | 303 | 222 | .016‡ | 253 | 185 | .711 |
| Total CD4+ T cells | 1662 | 789 | .001‡ | 451 | 1254 | .003‡ |
| Naive CD8+ T cells | 603 | 300 | .041‡ | 146 | 323 | .153 |
| Memory CD8+ T cells | 150 | 75 | .246 | 69 | 80 | .672 |
| Total CD8+ T cells | 697 | 552 | .412 | 419 | 640 | .328 |
| Total CD19+/CD20+ B cells | 677 | 381 | .059 | 70 | 649 | .005‡ |
| Total CD3-CD16/56+ NK cells | 193 | 127 | .014‡ | 119 | 129 | .625 |
| Ki-67 expression, % | ||||||
| Total CD4+ T cells | 3.0 | 6.1 | .07 | 6.4 | 5.8 | .25 |
| Naive CD4+ T cells | 1.1 | 2.6 | .24 | 3.2 | 2.0 | .27 |
| TRECs | ||||||
| Content in CD4+ T cells, no. per cell | 0.20 | 0.03 | .006‡ | 0.007 | 0.11 | .005‡ |
| Content in CD8+ T cells, no. per cell | 0.10 | 0.04 | .082 | 0.002 | 0.10 | .013‡ |
| No. in CD4+ T cells/μL blood | 410 | 23 | .001‡ | 1.4 | 105 | .002‡ |
| No. in CD8+ T cells/μL blood | 101 | 16 | .039‡ | 0.2 | 51 | .005‡ |
| Telomere length, relative to donor | ||||||
| Naive CD4+ T cells | ND | NA | NA | 1.3 | 1.6 | .079 |
| Memory CD4+ T cells | ND | NA | NA | 1.1 | 1.3 | .240 |
| Other parameters | ||||||
| Age of patient at HSCT, y | NA | 0.7 | 0.4 | 0.7 | .020‡ | |
| Age of donor at HSCT, y | NA | 24.2 | 15.9 | 27.9 | .457 | |
| SCID phenotype, no. | ||||||
| NK- | NA | 9 | 1 | 8 | .007‡ | |
| B- | NA | 6 | 5 | 1 | .025‡ | |
| B cells predominantly of donor origin, no. | NA | 11 | 4 | 7 | .578 | |
| NK cells predominantly of donor origin, no. | NA | 11 | 2 | 9 | .023‡ | |
| Monocytes predominantly of donor origin, no. | NA | 5 | 0 | 5 | .016‡ | |
| Myeloablative conditioning, BU/CY, no. | NA | 11 | 4 | 7 | .578 |
NK-cell chimerism in the group with poor long-term immune reconstitution was based on 7 patients because hardly any NK cells were present in one of the patients. For the SCID cohort, n = 19; for the group with poor reconstitution, n = 8; for the group with good reconstitution, n = 11.
BU indicates busulphan; CY, cyclophosphamide; ND, not done; NA, not applicable.
Median parameters of the whole SCID cohort at early follow-up (1-4 years after HSCT) were compared with age-matched healthy control subjects.
Within the SCID cohort, median parameters of immunity at early follow-up and HSCT-related variables were compared between the groups of patients with poor and good T-cell reconstitution at late follow-up.
Significant differences in P values.
Determinants of long-term T-cell reconstitution
Besides naive CD4+ T-cell counts and CD4+ TREC numbers, we investigated whether any of the other immune parameters at early follow-up or patient characteristics such as the type of SCID correlated with good or poor T-cell reconstitution at late follow-up (Table 2). Total CD4+ T-cell counts, CD19+/CD20+ B-cell counts, and TREC in CD8+ T cells at early follow-up were significantly lower in patients with poor long-term T-cell reconstitution than with good long-term T-cell reconstitution. Of note, naive CD4+ T-cell counts at 1 to 4 years after HSCT were significantly reduced in both groups of patients with SCID when compared with healthy age-matched control subjects (P < .001). At early follow-up there was no significant difference between both groups of patients in any of the CD8+ T-cell subsets, memory CD4+ T-cell counts, NK-cell counts, or Ki-67 expression (Table 2). Also, the number of nucleated cells in the graft did not differ between both groups of patients (data not shown). Remarkably, patients with poor T-cell reconstitution at late follow-up had received a transplant at a significantly younger age than patients with good long-term T-cell reconstitution. SCID phenotypes without NK cells (SCID NK–) correlated significantly with good outcome, whereas SCID types without B cells (SCID B–) correlated with poor T-cell recovery (Table 2). The number of reported clinical complications and infectious problems did not differ significantly between the 2 groups of patients (Table 1).
Longitudinal changes in naive CD4+ T-cell counts and CD4+ TREC numbers between early and late follow-up. Naive CD4+ T-cell counts (A) and CD4+ TREC numbers (B) were followed at early (1-4 years after HSCT) and late (> 5 years after HSCT) follow-up. Black circles and gray triangles denote patients with SCID with poor and good reconstitution of the naive CD4+ T-cell population at late follow-up, respectively. Open circles denote control values which were taken from age-matched (1-4.5 years and 5-32 years old, respectively) healthy individuals. The 2 open triangles in panel B represent the only child with good long-term immune reconstitution despite low TREC numbers at early follow-up. During early follow-up, this child was in an extremely poor clinical state and had malabsorption, which may explain the abnormal immune pattern at this time point.
Longitudinal changes in naive CD4+ T-cell counts and CD4+ TREC numbers between early and late follow-up. Naive CD4+ T-cell counts (A) and CD4+ TREC numbers (B) were followed at early (1-4 years after HSCT) and late (> 5 years after HSCT) follow-up. Black circles and gray triangles denote patients with SCID with poor and good reconstitution of the naive CD4+ T-cell population at late follow-up, respectively. Open circles denote control values which were taken from age-matched (1-4.5 years and 5-32 years old, respectively) healthy individuals. The 2 open triangles in panel B represent the only child with good long-term immune reconstitution despite low TREC numbers at early follow-up. During early follow-up, this child was in an extremely poor clinical state and had malabsorption, which may explain the abnormal immune pattern at this time point.
Despite the numerous significant correlations between early parameters and T-cell reconstitution at late follow-up, hardly any of these parameters could be used for early identification of individual patients at risk of poor long-term T-cell reconstitution. For example, 3 individuals who developed good T-cell reconstitution at late follow-up had naive CD4+ T-cell counts at early follow-up in the same range as individuals with poor long-term T-cell reconstitution (Figure 2A). Similarly, a clear distinction between individuals with poor and good long-term immune reconstitution could not be made on the basis of total CD4+ T-cell counts or CD19+/CD20+ B-cell counts at early follow-up, age at transplantation, or SCID phenotype (data not shown). The parameters that most clearly allowed for discrimination between individuals of the 2 groups of patients at early follow-up were the CD4+ TREC content and number (Figure 2B). Of the few patients in whom telomere length could be measured at early follow-up, naive CD4+ T-cell telomere lengths tended to be shorter in patients with poor (n = 2) long-term T-cell reconstitution than with good (n = 7) long-term T-cell reconstitution (Table 2).
Discussion
In our cohort of 19 patients with SCID, we found no evidence for an accelerated decline of T-cell immunity, thymus output, or telomere length with time after HSCT. In more than half of the patients, immune parameters at long-term follow-up were comparable to healthy age-matched control subjects, even up to 25 years after HSCT, whereas in others who showed poor T-cell reconstitution at the long term, these immune parameters turned out to be already low early after HSCT. Thus, our data do not confirm the suggestion put forward by Sarzotti et al14 and Patel et al15 that T-cell immunity in patients with SCID who receive a transplant may be impaired later in life because of long-term graft failure or an increased rate of deterioration of thymus function. Our study points out that on average long-term T-cell immunity in patients with SCID who receive a transplant may indeed not be as good as in healthy age-matched control subjects, but that this is merely due to a subset of patients who already reconstituted poorly early after HSCT. Sarzotti et al14 previously reported that after an initial normalization of T-cell diversity in patients with SCID during the first year after HSCT, T-cell diversity generally decreased during the next 10 years, concomitant with decreasing TREC values and naive T-cell numbers. This was interpreted as an indication that thymus output deteriorated at a faster pace in patients with SCID who received a transplant compared with healthy control subjects.14 Of note, however, the decrease in T-cell diversity that was reported was restricted to the memory CD8+ T-cell population and may thus be related to antigen-driven clonal expansion rather than decreasing thymus output.
Patel et al15 have performed a cross-sectional study on thymus function in a cohort of patients with SCID, of whom most had received a T-cell–depleted bone marrow graft from a haploidentical donor. No chemotherapy before transplantation and no GvHD prophylaxis were given. The percentage of patients with subnormal PBMC TREC contents early (1-4 years) and late (> 5 years) after HSCT was 18% (5 of 28 cases) and 32% (7 of 22 cases), respectively. The low frequency of patients with low TREC contents early after HSCT, compared with our study cohort (42%, 8 of 19), might be related to the relatively high proportion of patients with SCID-X1 in that study, the lower age at transplantation, and the fact that neither chemotherapy nor immunosuppressive treatment was applied, which is likely to have an effect on thymus function. The possible effect of myelosuppressive or myeloablative conditioning on the engraftment of long-term progenitors remains a matter of debate. In this respect, it is interesting that Patel et al15 observed a rapid decline of PBMC TREC contents in patients with SCID who received a transplant without conditioning, whereas such an accelerated decrease was not observed in the present study.
It is generally thought that T-cell immunity normalizes within one year after HSCT in most patients with SCID. However, in comparison with age-matched healthy control subjects, naive CD4+ T-cell counts in patients with SCID were still significantly reduced at a median follow-up of 1.5 years after HSCT, both in patients who did and who did not reconstitute well at the long term. In this respect, our data suggest that in a subgroup of patients with SCID, further improvement of T-cell recovery may occur relatively late after HSCT, differentiating the statement7 that the absence of normal T-cell function at least 2 years after HSCT could be an indication for a second transplantation procedure. Remarkably, a young age of the patients at the time of HSCT did not predict good long-term T-cell reconstitution in our cohort. However, it was previously found that thymus-dependent recovery of naive T cells 1 to 2 years after HSCT is superior in patients who received a transplant in the neonatal period.9 The observation that the SCID NK– phenotype correlated with good long-term T-cell reconstitution supports the concept that host NK cells may interfere with the development of lymphoid precursors and, thereby, impair the recovery of the immune system.8 However, our observation that the SCID B+ phenotype correlated with good outcome confirms previous studies that showed faster normalization of T- and B-cell function in B+ compared with B– SCID after HSCT.5,7 In this respect, it is of note that 4 of 5 patients with SCID-X1 (common γ-chain defect) are within the group with good T-cell reconstitution. However, overall, we did not find specific genetic defects to be related with good or poor T-cell reconstitution after HSCT, because of the heterogeneity of our SCID population, the small number of patients per genetic defect, and an unknown defect in 7 cases.
Our analysis demonstrated that CD4+ TREC contents and TREC numbers early after HSCT were predictive for long-term T-cell reconstitution. Decreased TREC contents at short-term follow-up in our study may be due to a lack of thymus output or to an increased rate of T-cell proliferation, possibly related to GvHD or infections.23,24 Because we found also that the total number of CD4+ TRECs per microliter of blood was decreased in patients with poor T-cell reconstitution, and that Ki-67 expression at early follow-up did not differ between patients with good and poor long-term T-cell reconstitution, we conclude that thymus output early after HSCT plays a crucial role in long-term T-cell reconstitution. Differences in thymus output between patients might be due to differences in the capacity of the originally vestigial thymus to be repopulated by progenitor cells, to differences in the quality and quantity of donor BM-derived progenitor cells, or to their capacity to repopulate the precursor compartment early after HSCT.13,25,26 The observation that T-cell reconstitution after HSCT correlated well with B-cell reconstitution would favor the latter 2 explanations, although we cannot exclude the possibility that poor maturation of naive B cells in secondary lymphoid organs was a direct consequence of poor T-cell reconstitution.
Taken together, our study shows that stable and long-lasting T-cell reconstitution is to be expected in patients with SCID who reconstitute well early after HSCT. The group of patients with SCID as a whole seems to have an increased risk of autoimmune or infectious problems at older age compared with other patients who receive HSCT. Unexpectedly, in our study cohort these problems were not clearly associated with the investigated immunologic parameters (see also Laffort et al27 ), which complicates clinical decision making on additional treatment. Previous studies have shown, however, that T-cell reconstitution is strongly associated with survival.7 Our analysis shows that TREC values measured at about 2 years after HSCT may, therefore, provide an important criterion when considering retransplantation or boost with donor stem cells in patients who do not reconstitute well at the short term. This strategy has been shown to be successful in 3 of 4 patients who received a second transplant 3 to 5 years after the first HSCT.7 However, the recently published failure of gene therapy for SCID-X1 at older age may point to age constraints on the regenerative capacity of the hypoplastic thymus of patients with SCID.28 These constraints should be taken into account when considering a second transplant with the purpose to restore the T-cell pool.
Prepublished online as Blood First Edition Paper, March 30, 2006; DOI 10.1182/blood-2006-01-009241.
Supported by an EC Biomed grant (BMH4-CT98-3007) to the European Society for Immunodeficiencies, by the Netherlands Organisation for Scientific Research (grant 916.36.003), and by the Dutch Cancer Society (grant 98-1825).
Laboratory experiments were performed by M.D.H., H.R., and S.A.O. R.G.B. and J.M.V. were responsible for patient care. Routine clinical laboratory data were produced under the supervision of M.J.v.T., and data management was performed by E.C.J.-Z. and J.H. T.W.K. collected material from healthy control subjects. J.A.B. and R.G.B. undertook the biostatistical analyses. J.M.V., R.G.B., and M.J.T. were responsible for the general design of the study, and M.D.H., F.M., H.R., and W.E.F. were responsible for the design of the specific parts on TRECs and telomere lengths, respectively. Together with J.A.B. these authors were involved in the interpretation of the results and general outline of the paper. J.A.B. and M.J.T. wrote the article.
J.A.B. and R.G.B. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Nienke Vrisekoop for useful comments to the manuscript, and Monique ten Dam and Jacqueline Waaijer for immunophenotyping.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal