Iatrogenic transmission by blood transfusion has been described in cases of human variant Creutzfeldt-Jakob disease (vCJD), experimental ovine bovine spongiform encephalopathy (BSE), and natural sheep scrapie, demonstrating that blood in these prion diseases is infectious. However, the possible effect of the transfusion, derived from differences in the inoculum (blood) and the route of infection (intravenous), on the pathologic phenotype of the disease in the recipients is not known. This study describes the neuropathologic phenotype of PrPd accumulation in sheep succumbing to neurologic disease after blood transfusion from donors experimentally infected with BSE; these were either clinically or subclinically affected at the time of donation. We demonstrate that blood can become infectious at early stages of ovine BSE infection and that the PrPd immunohistochemical phenotype is maintained after transfusion. This suggests that a change in the pathologic phenotype of vCJD would not be expected as a result of exposure to infected blood.
Introduction
Transmissible spongiform encephalopathies (TSEs), which include variant Creutzfeldt-Jakob disease (vCJD) in humans, scrapie in sheep and goats, and bovine spongiform encephalopathy (BSE) in cattle, are characterized by the accumulation of an abnormal, disease-associated isoform of the prion protein (PrPsc or, more generically, PrPd) in the brain of affected animals. However, widespread PrPd accumulation throughout lymphoreticular system (LRS) tissues is consistently observed in experimental sheep BSE1,2 and in natural scrapie,3-5 suggesting the possibility that blood could be infectious in such diseases. In ruminants, this has been clearly demonstrated for both scrapie and BSE by blood transfusion.6,7 Likewise, the consistent detection of PrPd in LRS tissues of vCJD patients8,9 has been followed by reports of 2 probable transmissions of the disease by blood transfusion from asymptomatic donors.10,11
One important aspect of these iatrogenic forms of TSEs is to ascertain whether the clinico-pathologic phenotype of the disease is altered after blood transfusion, as a result of change in the route of exposure and/or in the nature of the inoculum. Experimental sheep BSE can be distinguished from scrapie by the phenotype of PrPd accumulation in the brain12,13 and by the different reactivity of intracellular PrPd, both in brain and LRS tissues, to a panel of N-terminal PrP antibodies.5,14,15 The main aim of the present study was to determine if these phenotypic properties of experimental sheep BSE were maintained after blood transfusion. Because of the etiologic link between BSE and vCJD,16 it is likely that other features are also common to both infections, and our results may therefore be relevant for the case ascertainment of vCJD resulting from blood transfusion.
Materials and methods
Animals and experimental procedures
Cheviot sheep, which were part of a blood transfusion experiment involving scrapie and BSE, as described in detail elsewhere,7 were subjected to immunohistochemical (IHC) examination as described below under “Phenotype of PrPd accumulation in the brain.” Although postmortem examinations have been performed in a total of 48 sheep so far, only those that developed clinical TSE were considered toward the assessment of the phenotype of PrPd accumulation in brain and LRS tissues, and only those infected with BSE are dealt with in the present report. There were 3 categories of sheep used: (1) BSE donors (BDs; n = 6), either of AHQ/AHQ (n = 5) or ARQ/ARQ (n = 1) PrP genotype (indicated as amino acid single letter code at positions 136, 154, and 171 of the PrP protein sequence), that had been orally dosed with 5 g of a cattle BSE brain homogenate at 265 to 311 days of age. Two of them (BD1 and BD2) donated blood at clinical stage of disease and the other 4 at different time points of the preclinical phase. Donor BD5 gave blood to a recipient that is still alive (BR5) and donor BD6, to a sheep that died from an intercurrent condition and was not confirmed as TSE (BR6). (2) BSE recipients (BR; n = 6), all of the ARQ/ARQ genotype, that received a single blood transfusion (either 400-450 mL whole blood or buffy coat extracted from 50 mL blood) at 191 to 426 days of age from animals of group 1. One of these sheep (BR4) received blood from a donor (BD4) that was killed 9 months after oral BSE dosing, at which time it did not show any clinical, pathological, or IHC evidence of TSE. (3) BSE-positive controls (n = 6), all of the ARQ/AHQ genotype, that had been intravenously inoculated with 20 mL of a 1% BSE cattle brain homogenate at ages ranging from 332 to 410 days.
The relationships between donors and recipients, the ages at which infections were performed, the clinical status of the donors at the time of transfusion, and the time points at which animals developed clinical signs and were killed are provided in Figure 1. All sheep were closely monitored and humanely killed once clinical signs were considered highly suggestive of TSE.
Phenotype of PrPd accumulation in the brain
Six coronal sections of the brain from the above sheep were fixed in formaldehyde, trimmed, and embedded in paraffin wax according to standard procedures. These samples were processed for PrPd detection by IHC with primary antibody R145, as described in detail previously.13,17 Sections from positive-control and negative-control tissue blocks were included in each IHC run to ensure consistency in the sensitivity and specificity of the IHC procedure, respectively. Slides were blind-coded and included a mixture of BSE and scrapie cases; all examinations were carried out independently by 2 pathologists.
The phenotype of PrPd accumulation in the brain of each animal was defined by the magnitude of total PrPd, its topographic distribution, and the PrPd profile, as described in detail previously.12,13,17 The PrPd profile addresses the magnitude of deposition of different morphologic and cell-associated PrPd types in 6 brain areas. Each PrPd type was subjectively scored from 0 to 3 in each of those areas and average values were obtained for the whole brain. The PrPd profile is the graphic representation of those values as a continuous line plot, which includes sequentially intracellular PrPd types, extracellular glia-associated PrPd, extracellular types in the neuropil, and finally ependymal and vascular PrPd.
Accumulation of PrPd in LRS tissues
The degree of LRS involvement in each sheep was determined by IHC with mAb R145 in palatine tonsil, mesenteric lymph node, and spleen, as a factor of the proportion of lymphoid follicles accumulating PrPd and of the level of PrPd in positive follicles. This was scored from 0 (absence of immunolabeling) to 5 (abundant PrPd associated with tingible-body macrophages in dark and light zones and with follicular dendritic cells), as detailed elsewhere.15 To avoid biases arising from variation in follicle size or in the site of PrPd accumulation, only follicles in which distinct light and dark zones could be seen were considered.
Individual profiles of PrPd accumulation in the brains of BSE donor and recipient sheep. Relationship between donors (D; open symbols) and recipients (R; filled symbols), indicating points of birth (triangles), infection (diamonds), appearance of clinical signs (circles), and necropsy (squares); numbers indicate days. Donors and recipients in italics are not considered in the estimation of incubation periods or in the average PrPd profiles of Figure 2. Note that BD4 did not show signs of TSE at the time of death, and that BD1 and BD2 donated blood when clinically affected, while all the other donors were preclinical at that time. PrPd types are as follows: ITNR indicates intraneuronal; ITAS, intrastrocytic; ITMG, intramicroglial; STEL, stellate; SBPL, subpial; SBEP, subependymal; PRVS, perivascular; PVAC, perivacuolar; PRCO, particulate-coalescing; LINR, linear; PNER, perineuronal; EPEN, ependymal; and VASC, vascular plaques. Note that BR3 and BR4 have been referred to previously as D505 and F19, respectively.6,7
Individual profiles of PrPd accumulation in the brains of BSE donor and recipient sheep. Relationship between donors (D; open symbols) and recipients (R; filled symbols), indicating points of birth (triangles), infection (diamonds), appearance of clinical signs (circles), and necropsy (squares); numbers indicate days. Donors and recipients in italics are not considered in the estimation of incubation periods or in the average PrPd profiles of Figure 2. Note that BD4 did not show signs of TSE at the time of death, and that BD1 and BD2 donated blood when clinically affected, while all the other donors were preclinical at that time. PrPd types are as follows: ITNR indicates intraneuronal; ITAS, intrastrocytic; ITMG, intramicroglial; STEL, stellate; SBPL, subpial; SBEP, subependymal; PRVS, perivascular; PVAC, perivacuolar; PRCO, particulate-coalescing; LINR, linear; PNER, perineuronal; EPEN, ependymal; and VASC, vascular plaques. Note that BR3 and BR4 have been referred to previously as D505 and F19, respectively.6,7
PrPd epitope mapping in brain and LRS
The assessment of the immunoreactivity of intracellular PrPd with a panel of antibodies (epitope mapping)5,15 was carried out in samples of obex and the 3 LRS tissues with PrP antibodies BG4, P4, 521, 505, and R145. The PrP epitope recognition specificity of these antibodies is described in detail in previous publications.15
Results
Within the context of the BSE experimental animals included in this report, 4 of the donors donated blood during the preclinical period and 2 after they had developed clinical signs (Figure 1). While the later transfusions resulted in TSE-confirmed disease in 3 of 3 recipients (BR1, BR2a, and BR2b), only 2 (BR3 and BR4) of 4 recipients of blood collected at preclinical stages have so far succumbed to BSE. One (BR6) of the other 2 was IHC negative for PrPd in brain and LRS tissues when dying from an intercurrent condition more than 80 months after transfusion, and the other (BR5) is still alive at 82 months after transfusion. The 2 successful preclinical transfusions were from blood collected at 320 days (BD3, which developed clinical signs 289 days after donation) and 286 days (BD4, which was culled at the time of blood donation) after oral challenge. The 2 unsuccessful transmissions were from blood collected at 663 days (BD5, which developed clinical signs 97 days later) and 347 days (BD6, which developed clinical BSE 1723 days later) after oral dosing. Incubation periods were very similar for the recipients of blood collected from clinically affected (522 to 584 days) or preclinically affected (528 and 602 days) BSE donors. Although the mean incubation periods were shorter for blood recipients than for orally infected donors (552 and 934 days, respectively), they were not statistically different due to the protracted incubation period of animal BD6 (2070 days).
Phenotype of PrPd accumulation in the brain
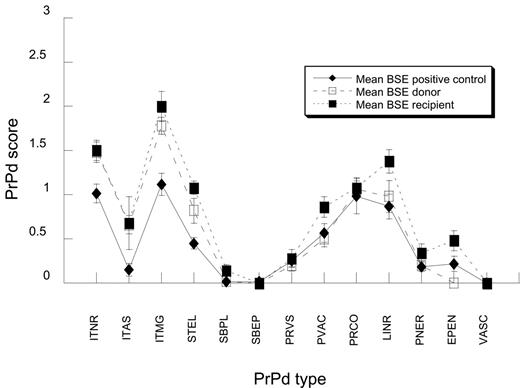
When data were analyzed by unpaired t tests, the recipients of blood from BSE-infected sheep showed a significantly higher magnitude of total PrPd accumulation in the brain (average score, 9.8; 95% confidence interval, 7.9-11.6) than the clinically affected BSE donors (average score, 7.6; 95% confidence interval, 6.3-9.0; P < .05) and than the BSE-positive controls (average score, 5.8; 95% confidence interval, 3.7-7.8; P < .01). All examined brain areas showed PrPd accumulations, and, while these were slight in the cerebral cortex, they reached similar moderate levels in all other areas examined (corpus striatum, thalamus/hypothalamus, midbrain, cerebellum, and obex). The PrPd profiles of BSE donors and recipients were very similar, both when compared individually (Figure 1) or as groups (Figure 2). They were also indistinguishable from those of the BSE-positive controls (Figure 2), and consistent with those previously described for BSE intracerebrally challenged animals of different PrP genotypes.13 The PrPd profile of BSE-infected sheep was characterized by moderate to high intracellular granular immunolabeling in neurons and microglial cells and moderate deposits in the extracellular neuropil in the form of linear and particulate or coalescing PrPd aggregates. In contrast, astrocyte-associated PrPd accumulations, both intracellular and extracellular (subpial, subependymal, and perivascular), were low or negligible, and vascular PrPd plaques were absent (Figure 2).
Accumulation of PrPd in LRS tissues
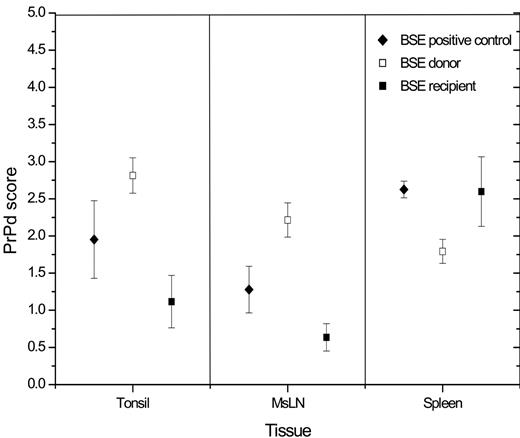
Immunohistochemistry with PrP antibody R145 revealed the presence of specific immunolabeling to a variable degree (Figure 3) in all LRS tissues examined from all BSE-infected sheep, except for BSE donor BD4 and BSE recipient BR6. When data were analyzed by unpaired t tests, blood recipients appeared to accumulate significantly lower levels of PrPd than orally infected donors in palatine tonsil (average, 1.1 and 2.8, respectively; P < .05) and mesenteric lymph node (average, 0.6 and 2.2, respectively; P < .05), but not in spleen (average 2.6 and 1.8, respectively, P > .05). BSE-positive controls showed intermediate levels of PrPd immunolabeling (Figure 3) with average values of 1.95, 1.28, and 2.62 for palatine tonsil, mesenteric lymph node, and spleen, respectively.
PrPd epitope mapping in brain and LRS
BSE-affected sheep, regardless of their group, failed to show intraneuronal and intramicroglial PrPd with P4 antibody. The intramicroglial labeling was also absent with polyclonal antibody 521. Intracellular accumulation of PrPd in macrophages was seen in all positive lymphoid follicles when IHC was done with C-terminal antibody R145, but was not discernible in serial sections incubated with N-terminal antibodies BG4, P4, 521, and 505. These results clearly demonstrated that the N-terminal truncation properties of BSE-derived PrPd remained unchanged after blood transfusion.
Average PrPd profiles of BSE. The profiles of BSE-positive controls, donors, and recipients are essentially the same. Results are expressed as means for each PrPd type and standard errors. PrPd types are as per legend of Figure 1.
Average PrPd profiles of BSE. The profiles of BSE-positive controls, donors, and recipients are essentially the same. Results are expressed as means for each PrPd type and standard errors. PrPd types are as per legend of Figure 1.
Magnitude of PrPd accumulation in LRS tissues. BSE donor sheep showed the highest levels of PrPd accumulation in palatine tonsil and MsLN but the lowest in the spleen. LN indicates lymph node. Results are expressed as mean and standard deviation for each LRS tissue.
Magnitude of PrPd accumulation in LRS tissues. BSE donor sheep showed the highest levels of PrPd accumulation in palatine tonsil and MsLN but the lowest in the spleen. LN indicates lymph node. Results are expressed as mean and standard deviation for each LRS tissue.
Discussion
Blood from preclinical and clinical BSE donors is infectious
Infectivity in the blood of sheep affected with natural scrapie and experimental ovine BSE has been demonstrated by means of blood transfusion.6,7 Within the context of the same experiments, the present report establishes that transmission can occur with blood collected from animals that show no accumulation of PrPd elsewhere (eg, BD4). These findings reinforce the view that infectivity in blood may precede abnormal PrP deposition in brain or in viscera. However, while the incubation periods in recipients of blood from preclinical donors were similar to those from clinically affected ones, the rate of successful transmissions for the whole of the experiment was higher from clinically affected BSE donors (3/5 = 60%) than from preclinical ones (3/19 = 16%, data not shown). While these results might suggest that infectivity in blood increases during the course of oral BSE infection, the failure of some transmissions could also be explained by PrP genetic factors in the donors and/or recipients. The latter possibility is under investigation (Wilfred Goldmann, personal oral communication, October 2005).
Transmission of vCJD from preclinical, asymptomatic donors is highly likely to have occurred, and the identification of such events has been possible only after the development of clinical disease10 or the detection of abnormal PrPd 11 in donors and/or recipients. If the scenario for vCJD was similar to that for experimental sheep BSE, it would be possible that other transmissions through blood transfusion may have occurred but have been overlooked in the absence of overt clinical disease or postmortem examinations.
Changes in the inoculum and route of infection do not obscure strain properties
The consistency of the pathologic phenotype of experimental sheep BSE across different breeds and PrP genotypes has been documented for intracerebral infections.13,15 Our results from PrPd profiling and PrPd epitope mapping in brain and LRS tissues confirm that the pathological phenotypes of sheep BSE after oral dosing with brain homogenate and after blood transfusion are indistinguishable. If, as is likely to be the case, such consistency applies to vCJD, the phenotypic assessment of the disease in humans may not resolve the origin of the infection: oral exposure or blood transfusion. The difference in the magnitude of total PrPd accumulation in the brains of BSE donors and recipients is valid for group comparisons, but cannot be used to distinguish between individuals. In addition, the variation in magnitude observed in this study might not result just from differences in route of inoculation but in PrP genotype, as has been shown for intracerebrally infected animals13 and for experimental scrapie.17,18 The consistency of the PrPd phenotype of experimental sheep BSE across PrP genotypes and routes of infection suggests that case ascertainment of vCJD will not be obscured by phenotypic changes that might potentially arise from these variables or from clinical syndromes mimicking other prion diseases.
The lower degree of PrPd accumulation observed in the mesenteric lymph node and palatine tonsil of the blood recipients compared with the orally dosed BSE donors is probably related to the route of infection. These differences, however, are only quantitative and therefore unlikely to be of use to distinguish between routes of infection at the individual animal or patient level.
Despite individual variability, BSE-affected sheep, either donors or recipients, could be distinguished from scrapie-affected sheep (data not shown) by the phenotype of PrPd accumulation in the brain and by the immunoreactivity of PrPd to N- and C-terminal antibodies. Our results on epitope mapping not only confirm previous observations that the intracellular truncation of BSE-derived PrPd differs from that of scrapie-derived PrPd,5,15 but also indicate that this property is not altered after blood transfusion.
Usefulness of the experimental sheep BSE model
The demonstration of infectivity in the blood of preclinical BSE- and scrapie-infected sheep agrees with preliminary data suggesting that vCJD can be transmitted by blood transfusion from donors incubating the disease. These results indicate the urgent need to develop a diagnostic test for human blood samples in order to identify potentially contaminated blood supplies, to establish which blood fractions or preparations are infectious, and to correlate infectivity with detection of PrPd. These are, however, very sensitive issues, and the validation of any diagnostic techniques, several of which are now being developed,19 will require meticulous and comprehensive approaches. Due to its parallelism with vCJD, the experimental sheep BSE model offers great potential for such validation, although caution must be taken when extrapolating results from animal models into humans. This model allows blood collection from donors and recipients at different time points during preclinical, clinical, and terminal stages of disease, so that data on blood infectivity and test sensitivity can be correlated. Further blood transfusion studies from sheep at preclinical stage could provide a better assessment of the temporal evolution of disease in relation to blood tests results.
Prepublished online as Blood First Edition Paper, March 28, 2006; DOI 10.1182/blood-2005-12-5156.
Supported in part by the UK Department of Health, the Biotechnology and Biological Sciences Research Council (BBSRC), and Department of Environmental, Food, and Rural Affairs (DEFRA).
S.S. examined the tissues, analyzed data, and wrote the report; L.G. designed the study, examined tissues, coordinated data analysis, and wrote the report;
F.H. carried out the blood transfusions and analyzed data; N.H. initiated the transfusion study, analyzed data, and reviewed the report; S.M. performed immunohistochemical examination of positive-control samples to ensure consistency of immunohistochemistry; and M.J. contributed to the study planning and reviewed the report.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Technical work on immunohistochemistry by Hazel Baird, Lynn Fairlie, Ann Dunachie, and Maria Oliva and technical support on graphics by Janey Barns (VLA, Lasswade) are acknowledged. Assistance with blood collection was given by Calum McKenzie, James Foster, and David Parnham (IAH). Data checking had the assistance of Sue Halliday and Sandra McCutcheon (IAH). The authors greatly appreciate Jim Hope's (VLA) critical appraisal of the paper and Wilfred Goldmann's (IAH) contribution to discussion of the sheep genetics.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal