Although adult mouse hematopoietic stem cells (HSCs) have been purified to near homogeneity, it remains impossible to achieve this with fetal HSCs. Adult HSC purity recently has been enhanced using the SLAM family receptors CD150, CD244, and CD48. These markers are expressed at different stages of the hematopoiesis hierarchy, making it possible to highly purify adult HSCs as CD150+CD48–CD244– cells. We found that SLAM family receptors exhibited a similar expression pattern in fetal liver. Fetal liver HSCs were CD150+CD48–CD244–, and the vast majority of colony-forming progenitors were CD48+CD244–CD150– or CD48+CD244+CD150–, just as in adult bone marrow. SLAM family markers enhanced the purification of fetal liver HSCs. Whereas 1 (11%) of every 8.9 ThylowSca-1+lineage–Mac-1+ fetal liver cells gave long-term multilineage reconstitution in irradiated mice, 1 (18%) of every 5.7 CD150+CD48–CD41– cells and 1 (37%) of every 2.7 CD150+CD48–Sca-1+lineage–Mac-1+ fetal liver cells gave long-term multilineage reconstitution. These data emphasize the robustness with which SLAM family markers distinguish progenitors at different stages of the hematopoiesis hierarchy and enhance the purification of definitive HSCs from diverse contexts. Nonetheless, CD150, CD244, and CD48 are not pan-stem cell markers, as they were not detectably expressed by stem cells in the fetal or adult nervous system.
Introduction
Definitive hematopoietic stem cells (HSCs) first arise in mice in the aorta-gonad-mesonephros (AGM) region and perhaps in other vascular niches around embryonic day 10 (E10).1-3 Soon thereafter definitive hematopoiesis is initiated in the fetal liver and placenta, the major hematopoietic organs during midgestation.4,5 Although hematopoiesis in the placenta declines after E13.5, hematopoiesis continues at high levels in the liver until after birth.
HSCs are highly enriched in the ThylowSca-1+lineage–Mac-1+ fraction of fetal liver cells. These cells represent 0.04% of E12.5 to E14.5 fetal liver cells, and 1 (13%) of every 7.8 intravenously injected ThylowSca-1+lineage–Mac-1+ cells were observed to engraft in irradiated mice and give long-term multilineage reconstitution.6 Similar enrichments of HSC activity have been obtained using slightly different combinations of markers.7 This had been thought to be near purity, but adult bone marrow HSCs recently have been purified to the point that at least 40% (1 in 2.5) of single cells from various populations give long-term multilineage reconstitution in irradiated mice.8-11 The enhanced purification of adult HSCs has demonstrated that these cells are capable of engrafting efficiently after transplantation into irradiated mice and has increased the precision with which adult HSCs can be studied. These observations raise the question of whether fetal HSCs also can engraft highly efficiently after transplantation and whether it would be possible to enhance their purification with new markers.
There are a number of pronounced phenotypic and functional differences between fetal and adult HSCs. Fetal liver HSCs divide rapidly and give more robust and rapid reconstitution of irradiated recipients relative to adult HSCs.6,12 Fetal liver HSCs differ from adult bone marrow HSCs in the expression of specific markers such as Mac-1, CD144, and AA4.16,13,14 as well as in their general gene expression profile.15-17 There also are clear differences between fetal and adult HSCs in the regulation of basic stem cell properties such as self-renewal and developmental potential. For example, fetal and adult HSCs differ in their dependence on polycomb family members that regulate self-renewal, including Bmi-1,18 Mel-18,19 and Rae-28.20 Fetal liver HSCs have the capacity to form certain subtypes of B and T cells that adult HSCs are unable to form, even when transplanted into the fetal environment.21-23 These observations demonstrate that fetal liver HSCs are phenotypically and functionally distinct from adult HSCs.
SLAM family receptors recently were found to be differentially expressed among primitive progenitors in adult mouse bone marrow and cytokine mobilized spleen: CD150 was expressed by HSCs but not multipotent progenitors (MPPs) or restricted progenitors, whereas CD244 was expressed by at least some transiently reconstituting MPPs but not by HSCs, and CD48 was expressed by most colony-forming restricted progenitors but not by HSCs or MPPs.11,24 These SLAM family members were so precisely differentially expressed that it was possible to highly purify HSCs using a simple combination of SLAM family members. Twenty percent of CD150+CD48– cells and 45% of CD150+CD48–CD41– cells purified from adult bone marrow gave long-term multilineage reconstitution upon transplantation into irradiated mice.11 These observations raise 2 important questions in the context of fetal hematopoiesis. First, do SLAM family receptors exhibit a similar pattern of expression on fetal hematopoietic progenitors? If so, this would further emphasize the robustness with which these receptors mark progenitors that differ according to primitiveness. Second, can these receptors also be used to enhance the purification of fetal liver HSCs? If so, these new markers would enhance the precision with which fetal HSCs can be studied.
Materials and methods
Flow-cytometric isolation of stem and progenitor cells
All mice used in this study were housed in the Unit for Laboratory Animal Medicine at the University of Michigan. Fetal livers were isolated from E14.5 C57BL/Ka-CD45.2:Thy-1.1 embryos. Cells were triturated with Hanks buffered salt solution (HBSS) without calcium or magnesium, supplemented with 2% heat-inactivated calf serum (Gibco, Grand Island, NY; HBSS+) and filtered through nylon screen (45 μM; Sefar America, Kansas City, MO) to obtain a single cell suspension.
Fetal liver HSCs were isolated as previously described.6 Whole fetal liver cells were incubated with unconjugated monoclonal antibodies to lineage markers including B220 (6B2), CD3 (KT31.1), CD5 (53-7.3), CD8 (53-6.7), Gr-1 (8C5), and Ter119. Following dilution, pelleted cells were resuspended in anti–rat IgG specific F(ab)2 conjugated to phycoerythrin (PE; Jackson ImmunoResearch, West Grove, PA). Cells then were stained with directly conjugated antibodies to Mac-1 (M1/70-allophycocyanin [APC]), Sca-1 (Ly6A/E-biotin), Thy-1.1 (19XE5-florescein isothiocyanate [FITC]), CD48 (HM48-1-PE), and CD4 (GK1.5-PE; note that CD4 was considered part of the lineage cocktail and therefore is not listed separately), followed by staining with streptavidin conjugated to APC-Cy7 (PharRed; PR) (Becton Dickinson, San Jose, CA). Progenitors often were enriched by preselecting for Sca-1+ cells using paramagnetic microbeads (Miltenyi Biotec, Auburn, CA) and autoMACS.
Cells sorted on the basis of CD150 expression were incubated with unconjugated antibody to CD150 (26D12; DNAX, Palo Alto, CA) and subsequently stained with goat anti–rat IgG F(ab)2 fragment conjugated to FITC, PE, or APC (Jackson ImmunoResearch). When CD150 was combined with lineage markers, antibodies against lineage markers were directly conjugated to fluorochromes. Cells sorted according to CD41, CD48, or CD244 expression were stained with directly conjugated anti-CD41 (MWReg30-FITC), anti-CD48 (HM48-1-FITC or PE), or anti-CD244 (2B4-FITC), respectively.
Six-color staining was used in some experiments to analyze the heterogeneity of CD150, CD48, and CD41 in the ThylowSca-1+lineage– Mac-1+ population. Whole fetal liver cells were incubated with FITC-conjugated anti-CD150, CD48 or CD41; PE-conjugated antibodies to lineage markers; anti–Mac-1 conjugated to APC; anti–Sca-1 conjugated to biotin; and anti–Thy-1.1 conjugated to PE-Cy5, followed by streptavidin conjugated to APC-Cy7. Cells were resuspended in 2 μg/mL 7-AAD (Molecular Probes, Eugene, OR) or DAPI to discriminate live from dead cells. All flow cytometry was performed on a FACSVantage SE-dual laser, 3-line flow cytometer (Becton Dickinson). An Enterprise II laser (Coherent, Santa Clara, CA) provided a primary 488-nm line and a separated 351-nm line (directed to the cytometer's tertiary laser position). The other laser, a Model 127 HeNe laser (Spectra-Physics, Mountain View, CA) provided a 633-nm line. Interlaser compensation was provided by OmniComp (Becton Dickinson).
Long-term competitive reconstitution assays
Adult recipient mice (8-week-old C57BL/Ka-CD45.1:Thy-1.2 mice) were irradiated with an Orthovoltage x-ray source (Philips, Eindhoven, The Netherlands) delivering approximately 300 rads per minute in 2 doses of 550 to 570rad each, delivered at least 2 hours apart. When small numbers of HSCs were tested for reconstituting potential, the relevant donor (CD45.2+) population was sorted, and the number of cells to be injected per mouse was resorted into individual wells of a 96-well plate containing 200 000 CD45.1+ whole bone marrow cells in HBSS+. The contents of individual wells were injected into the retro-orbital venous sinus of individual lethally irradiated CD45.1+ recipients. For at least 16 weeks after transplantation, blood was obtained from the tail veins of recipient mice, subjected to ammonium-chloride potassium red-cell lysis,25 and stained with directly conjugated antibodies to CD45.2 (104, FITC), B220 (6B2), Mac-1 (M1/70), CD3 (KT31.1), and Gr-1 (8C5) conjugated to PE or APC to monitor donor cell engraftment.
Results
SLAM receptor expression patterns in the fetal liver
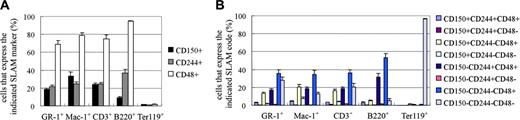
To begin to assess whether SLAM family receptors exhibit a similar expression pattern in fetal liver as compared to adult bone marrow, we examined the expression of CD150, CD244, and CD48 in the E14.5 fetal liver. 67% ± 3.8% of fetal liver cells were negative for CD150, CD244, and CD48 (Figure 1D). Consistent with this, Ter119+ erythroid cells, which account for most fetal liver cells, rarely expressed any of the SLAM family markers (Figure 2A). However, the less frequent myeloid (Gr-1+ or Mac-1+), T- (CD3+), and B-lineage (B220+) cells in the fetal liver sometimes expressed CD150 or CD244, and frequently expressed CD48 (Figure 2A,B). This is similar to what is observed in adult bone marrow, where CD48+ cells are much more frequent than CD150+ or CD244+ cells, and cells expressing differentiation markers are generally much more likely to express CD48 than CD150 or CD244.11,24
CD48 is expressed by restricted progenitors but not by HSCs in fetal liver
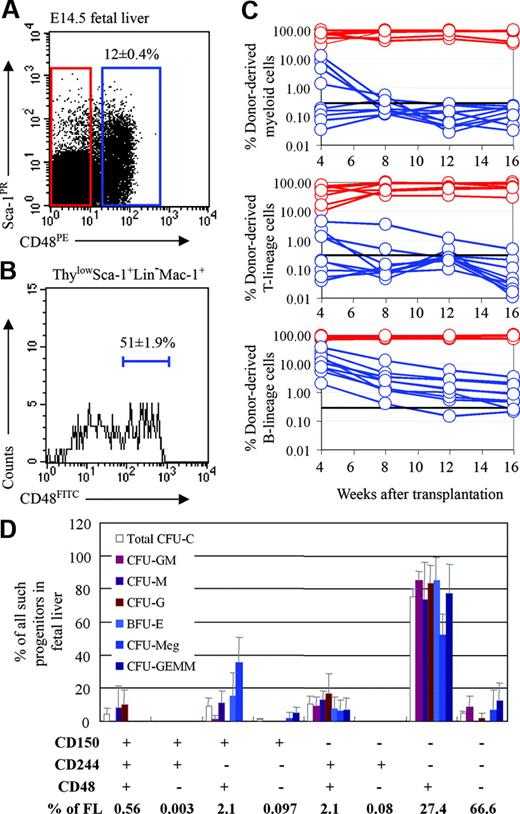
CD48 is expressed by most colony-forming progenitors in adult bone marrow, but not by HSCs or transiently reconstituting MPPs.11,24 In this study, we tested whether CD48 is also a marker of restricted progenitors during fetal development. CD48 is expressed by 12% ± 0.4% (mean ± SD) of E14.5 liver cells (Figure 1A). To test whether fetal liver HSCs are CD48+ or CD48–, we performed competitive reconstitution assays in which CD48+ or CD48– fetal liver cells were transplanted into lethally irradiated recipient mice along with a radioprotective dose of 200 000 recipient-type whole bone marrow cells. All recipients (7 of 7) that received CD48– cells in 2 independent experiments were long-term multilineage reconstituted (Figure 1C). Recipients that received CD48+ cells in the same experiments were never long-term multilineage reconstituted but were either transiently multilineage reconstituted (4 of 9) or transiently reconstituted in only the B-cell lineage (5 of 9) (Figure 1C). These results indicate that CD48 exhibits a similar expression pattern in fetal liver as compared to adult bone marrow to the extent that it is expressed on restricted B-cell progenitors but not by HSCs. It remains unclear from these data whether a subset of transiently reconstituting MPPs also expresses CD48 in fetal liver or whether the multilineage reconstitution of some recipients was caused by the combined activity of multiple restricted myeloid and lymphoid progenitors.
HSCs are contained within the CD48– fraction while colony-forming progenitors are almost entirely in the CD48+ fraction of fetal liver cells. (A) Twelve percent of fetal liver cells expressed CD48. (B) CD48 expression was heterogeneous in the ThylowSca-1+lineage–Mac-1+ HSC population, with 51% of cells being CD48+. For this analysis CD48, Thy-1, Sca-1, lineage marker, and Mac-1 staining were detected in the FITC, PE-Cy5, APC-Cy7 (PR), PE, and APC channels, respectively. (C) Recipient mice received transplants of 5750 CD48+ fetal liver cells (blue lines) or 44 250 CD48– fetal liver cells (red lines). Cell doses were based on the proportion of CD48+ versus CD48– cells in 50 000 fetal liver cells (which gives long-term multilineage reconstitution in nearly all recipient mice), as done in previous studies of marker expression by HSCs.6,14,24,26 Each line represents the frequency of donor-derived myeloid, B, or T cells in a single mouse. The black line at 0.3% represents the background threshold, meaning that reconstitution could not be detected in mice falling below this line. All long-term multilineage reconstituting activity was contained within the CD48– cell fraction (7 of 7 mice). CD48+ cells gave transient multilineage reconstitution (4 of 9 mice) or B-lineage-only reconstitution (5 of 9 mice). Data are from 1 of 2 independent experiments that gave similar results. (D) Fetal liver cells were divided based on their expression of all possible combinations of the CD48, CD244, and CD150 SLAM family receptors and sorted into methylcellulose to measure colony-forming activity. Each bar represents the percentage of all colony-forming progenitors of each type that fell within the indicated cell fraction. For example, around 80% of all CFU-GM, CFU-G, and CFU-M in the fetal liver fell within the CD48+CD244–CD150– cell population. Error bars indicate SD.
HSCs are contained within the CD48– fraction while colony-forming progenitors are almost entirely in the CD48+ fraction of fetal liver cells. (A) Twelve percent of fetal liver cells expressed CD48. (B) CD48 expression was heterogeneous in the ThylowSca-1+lineage–Mac-1+ HSC population, with 51% of cells being CD48+. For this analysis CD48, Thy-1, Sca-1, lineage marker, and Mac-1 staining were detected in the FITC, PE-Cy5, APC-Cy7 (PR), PE, and APC channels, respectively. (C) Recipient mice received transplants of 5750 CD48+ fetal liver cells (blue lines) or 44 250 CD48– fetal liver cells (red lines). Cell doses were based on the proportion of CD48+ versus CD48– cells in 50 000 fetal liver cells (which gives long-term multilineage reconstitution in nearly all recipient mice), as done in previous studies of marker expression by HSCs.6,14,24,26 Each line represents the frequency of donor-derived myeloid, B, or T cells in a single mouse. The black line at 0.3% represents the background threshold, meaning that reconstitution could not be detected in mice falling below this line. All long-term multilineage reconstituting activity was contained within the CD48– cell fraction (7 of 7 mice). CD48+ cells gave transient multilineage reconstitution (4 of 9 mice) or B-lineage-only reconstitution (5 of 9 mice). Data are from 1 of 2 independent experiments that gave similar results. (D) Fetal liver cells were divided based on their expression of all possible combinations of the CD48, CD244, and CD150 SLAM family receptors and sorted into methylcellulose to measure colony-forming activity. Each bar represents the percentage of all colony-forming progenitors of each type that fell within the indicated cell fraction. For example, around 80% of all CFU-GM, CFU-G, and CFU-M in the fetal liver fell within the CD48+CD244–CD150– cell population. Error bars indicate SD.
To test whether other restricted fetal progenitors express CD48, we fractionated fetal liver according to the differential expression of SLAM family receptors and examined their ability to form colonies in methylcellulose (Figure 1D). Virtually all colony-forming activity from the fetal liver was present in CD48+ cell populations, as we had previously observed in adult bone marrow.11 75.6% ± 4.5% of all colony-forming progenitors (Total CFU-C, Figure 1D) were contained within the CD48+CD244–CD150– subset of cells that accounted for 27.4% ± 4.0% of all fetal liver cells. Another 10.3% ± 4.8% of colony-forming progenitors fell within the CD48+CD244+CD150– population that accounted for 2.2% ± 0.9% of fetal liver cells. Notably, the 67% of fetal liver cells that lacked the expression of any of these SLAM family markers exhibited little colony-forming activity (Figure 1D). Thus, the expression pattern of SLAM family receptors on colony-forming progenitors in the fetal liver resembled the pattern previously observed in adult bone marrow, with the vast majority of colony-forming progenitors falling within the CD48+ CD244–CD150– and CD48+CD244+CD150– fractions of cells. One difference, however, is that while most colony-forming progenitors in adult bone marrow are CD244+, most colony-forming progenitors in fetal liver were CD244–.
CD48 enhances the purification of fetal liver HSCs
To test whether the use of CD48 could enhance the purification of fetal liver HSCs, we examined its expression pattern within the ThylowSca-1+lineage–Mac-1+ cell population that is highly enriched for fetal liver HSCs.6 Fetal liver ThylowSca-1+ lineage–Mac-1+ cells were heterogeneous, with 51% ± 1.9% of these cells being CD48+ (Figure 1B). Since CD48+ fetal liver cells lacked HSC activity (Figure 1C), this suggested that fetal liver HSCs might be more highly enriched by selecting the CD48– subset of ThylowSca-1+lineage–Mac-1+ cells. To test this, we sorted the CD48+ and CD48– subsets of Sca-1+lineage–Mac-1+ fetal liver cells and injected 10 cells from either population into irradiated mice along with a radioprotective dose of 200 000 recipient bone marrow cells (Table 1). As expected, most recipients (3 of 4) of Sca-1+lineage–Mac-1+CD48– cells were long-term multilineage reconstituted. None of the recipients (0 of 8) of Sca-1+lineage–Mac-1+CD48+ cells showed detectable engraftment in any hematopoietic lineage. The absence of HSC activity in the CD48+ fraction of Sca-1+lineage–Mac-1+ cells further demonstrates that fetal liver HSCs, like adult HSCs, are CD48–.
Competitive reconstitution of irradiated mice with various fetal liver HSC populations reveals that CD48 enhances the purity of fetal liver HSCs
Donor cells . | Cell dose . | Mice with engrafted cells . | Frequency of engrafted cells . | Mice with long-term multilineage reconstitution . | Frequency of mice that were long-term multilineage reconstituted (%) . |
|---|---|---|---|---|---|
| TSLM | 3 | 4 of 10 | 1 in 6.4 | 3 of 10 | 1 in 8.9 (11) |
| CD48- SLM | 10 | 3 of 4 | 1 in 7.7 | 3 of 4 | 1 in 7.7 (13) |
| CD48+ SLM | 10 | 0 of 8 | NA | NA | NA |
| CD48- TSLM | 3 | 15 of 21 | 1 in 2.9 | 10 of 21 | 1 in 5.2 (19) |
| CD48- TSLM | 1 | 9 of 14 | 1 in 1.6 | 5 of 14 | 1 in 2.8 (36) |
Donor cells . | Cell dose . | Mice with engrafted cells . | Frequency of engrafted cells . | Mice with long-term multilineage reconstitution . | Frequency of mice that were long-term multilineage reconstituted (%) . |
|---|---|---|---|---|---|
| TSLM | 3 | 4 of 10 | 1 in 6.4 | 3 of 10 | 1 in 8.9 (11) |
| CD48- SLM | 10 | 3 of 4 | 1 in 7.7 | 3 of 4 | 1 in 7.7 (13) |
| CD48+ SLM | 10 | 0 of 8 | NA | NA | NA |
| CD48- TSLM | 3 | 15 of 21 | 1 in 2.9 | 10 of 21 | 1 in 5.2 (19) |
| CD48- TSLM | 1 | 9 of 14 | 1 in 1.6 | 5 of 14 | 1 in 2.8 (36) |
The indicated number of donor-type (CD45.2+) cells from fetal liver were transplanted intravenously into lethally irradiated adult recipients (CD45.1+) along with 200 000 recipient-type whole bone marrow cells for radioprotection. Recipients were considered engrafted by donor cells if any CD45.2+ cells were detected in their peripheral blood (above background: > 0.3% of white blood cells). Mice were considered long-term multilineage reconstituted if donor-type myeloid, B, and T cells were present for more than 16 weeks after reconstitution. The frequency of HSCs was calculated by Poisson limit-dilution statistics6,26 based on the frequency of mice that became long-term multilineage reconstituted.
TSLM indicates ThylowSca-1+lineage-Mac-1+ fetal liver cells; NA, not applicable.
To directly test whether the exclusion of CD48+ cells enhances the purity of fetal liver HSCs, we transplanted 3 or 1 ThylowSca-1+lineage–Mac-1+CD48– fetal liver cells into lethally irradiated recipient mice along with a radioprotective dose of 200 000 recipient-type whole bone marrow cells (Table 1). When CD48 was not used as a marker, 1 (11%) in 8.9 ThylowSca-1+lineage–Mac-1+ fetal liver cells (which represented 0.025% ± 0.002% of E14.5 fetal liver cells in these experiments) engrafted and gave long-term multilineage reconstitution, similar to what we reported previously.6 In contrast, an average of 1 (25%) in 4 ThylowSca-1+ lineage–Mac-1+CD48– fetal liver cells (which represented 0.011% ± 0.001% of E14.5 fetal liver cells) engrafted and gave long-term multilineage reconstitution. The data indicate that the purity of fetal liver HSCs can be increased by excluding contaminating CD48+ cells that lack detectable reconstituting activity.
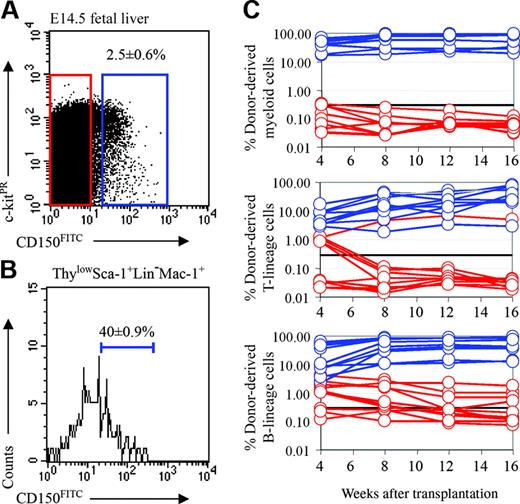
CD150 is expressed by fetal liver HSCs
We recently discovered that the founding member of the SLAM family, CD150, is a highly restricted marker of HSCs in both young and old adult mice.11,24 In the fetal liver, only 2.5% ± 0.6% of cells were CD150+ (Figure 3A). To test whether fetal liver HSCs are CD150+, we performed competitive reconstitution assays in which CD150+ or CD150– fetal liver cells were transplanted into lethally irradiated recipient mice along with a radioprotective dose of 200 000 recipient-type whole bone marrow cells (Figure 3C). All recipients (10 of 10) that received CD150+ cells were long-term multilineage reconstituted, but no recipients that received CD150– cells were long-term multilineage reconstituted. In contrast, recipients of CD150– cells were sometimes transiently reconstituted in the B and T lineages (4 of 10) or only in the B lineage (3 of 10). These data indicate that all fetal liver HSC activity is in the CD150+ cell fraction, as previously observed in adult mice.11,24
Ter119+ erythroid lineage cells rarely express CD150, CD244, or CD48, while most other myeloid, B-, and T-lineage cells in the fetal liver express CD48. In the E14.5 fetal liver most myeloid lineage (Gr-1+ or Mac-1+), T-lineage (CD3+), and B-lineage (B220+) cells express CD48, but only around 20% of such cells express CD150 and/or CD244 (A, B). In contrast, no more than rare Ter119+ erythroid cells express any of these receptors (A, B). Error bars indicate SD.
Ter119+ erythroid lineage cells rarely express CD150, CD244, or CD48, while most other myeloid, B-, and T-lineage cells in the fetal liver express CD48. In the E14.5 fetal liver most myeloid lineage (Gr-1+ or Mac-1+), T-lineage (CD3+), and B-lineage (B220+) cells express CD48, but only around 20% of such cells express CD150 and/or CD244 (A, B). In contrast, no more than rare Ter119+ erythroid cells express any of these receptors (A, B). Error bars indicate SD.
About 40% of ThylowSca-1+lineage–Mac-1+ cells expressed CD150 (Figure 3B). The heterogeneity of CD150 in this population raised the possibility that HSCs in fetal liver might be more highly purified using CD150. To test this, we sorted the CD150+ and CD150– subsets of Sca-1+lineage–Mac-1+ fetal liver cells and injected 10 cells from either population into irradiated mice along with a radioprotective dose of 200 000 recipient bone marrow cells (Table 2). Of 7 recipients of Sca-1+lineage–Mac-1+CD150+ cells, 4 were long-term multilineage reconstituted. However, just 1 of 7 recipients of Sca-1+lineage–Mac-1+CD150– cells showed long-term multilineage reconstitution. Of 7 recipients of Sca-1+ lineage–Mac-1+CD150– cells, 2 showed transient multilineage reconstitution, and another 3 recipients exhibited transient reconstitution in lymphoid lineages. This further demonstrated that most if not all fetal liver HSCs are CD150+, while many transiently reconstituting multipotent progenitors and restricted progenitors are CD150–, as observed in adult bone marrow.11
Competitive reconstitution of irradiated mice with various fetal liver HSC populations reveals that CD150 enhances the purity of fetal liver HSCs
Donor cells . | Cell dose . | Mice with engrafted cells . | Frequency of engrafted cells . | Mice with long-term multilineage reconstitution . | Frequency of mice that were long-term multilineage reconstituted (%) . |
|---|---|---|---|---|---|
| CD150-SLM | 10 | 6 of 7 | 1 in 5.7 | 1 of 7 | 1 in 65.4 (1.5) |
| CD150+SLM | 10 | 6 of 7 | 1 in 5.7 | 4 of 7 | 1 in 12.3 (8) |
| CD150+CD48-SLM | 1 | 9 of 17 | 1 in 1.9 | 7 of 17 | 1 in 2.4 (42) |
| CD150+CD48-SLM | 1 | 5 of 13 | 1 in 2.6 | 4 of 13 | 1 in 3.3 (30) |
| CD150+CD48-CD41- | 1 | 3 of 17 | 1 in 5.7 | 3 of 17 | 1 in 5.7 (18) |
| CD150+CD48-CD41- | 1 | 6 of 23 | 1 in 3.8 | 4 of 23 | 1 in 5.8 (17) |
Donor cells . | Cell dose . | Mice with engrafted cells . | Frequency of engrafted cells . | Mice with long-term multilineage reconstitution . | Frequency of mice that were long-term multilineage reconstituted (%) . |
|---|---|---|---|---|---|
| CD150-SLM | 10 | 6 of 7 | 1 in 5.7 | 1 of 7 | 1 in 65.4 (1.5) |
| CD150+SLM | 10 | 6 of 7 | 1 in 5.7 | 4 of 7 | 1 in 12.3 (8) |
| CD150+CD48-SLM | 1 | 9 of 17 | 1 in 1.9 | 7 of 17 | 1 in 2.4 (42) |
| CD150+CD48-SLM | 1 | 5 of 13 | 1 in 2.6 | 4 of 13 | 1 in 3.3 (30) |
| CD150+CD48-CD41- | 1 | 3 of 17 | 1 in 5.7 | 3 of 17 | 1 in 5.7 (18) |
| CD150+CD48-CD41- | 1 | 6 of 23 | 1 in 3.8 | 4 of 23 | 1 in 5.8 (17) |
The indicated number of donor-type (CD45.2+) cells from fetal liver were transplanted intravenously into lethally irradiated recipients (CD45.1+) along with recipient-type 200 000 whole bone marrow cells for radioprotection. Recipients were considered engrafted by donor cells if any CD45.2+ cells were detected in their peripheral blood (above background: > 0.3% of white blood cells). Mice were considered long-term multilineage reconstituted if donor-type myeloid, B, and T cells were present for more than 16 weeks after reconstitution. The frequency of HSCs was calculated by Poisson limit-dilution statistics6,26 based on the frequency of mice that became long-term multilineage reconstituted.
C150 and CD48 enhance the purity of fetal liver HSCs
The results suggested that the concomitant use of CD48 and CD150 might further enhance fetal liver HSC purity. To test this possibility, we competitively transplanted single CD150+CD48–Sca-1+ lineage–Mac-1+ fetal liver cells into lethally irradiated mice along with 200 000 recipient whole bone marrow cells (Table 2). The frequency of the CD150+CD48–Sca-1+lineage–Mac-1+ population was 0.011% ± 0.0015% of fetal liver cells. In 2 independent experiments, a total of 11 of 30 mice became long-term multilineage reconstituted by single CD150+CD48–Sca-1+lineage–Mac-1+ cells. This corresponds to 1 (37%) of every 2.7 cells in this population exhibiting HSC activity. This suggests that the combination of CD48 and CD150 with other HSC markers enhanced the purity of fetal liver HSCs by approximately 3-fold relative to the Thy-1lowSca-1+lineage–Mac-1+ cell population (1 in 8.9 cells gave HSC activity; Table 1). The functional purity of CD150+CD48–Sca-1+ lineage–Mac-1+ cells (1 in 2.4 to 1 in 3.3; Table 2) appeared comparable or slightly better than that of ThylowSca-1+lineage–Mac-1+CD48– cells (1 in 2.8 to 1 in 5.2; Table 1), which represented a similar fraction of fetal liver cells.
Whole bone marrow cells from 3 primary recipients that had been reconstituted for 6 months by single CD150+CD48–Sca-1+ lineage–Mac-1+ donor cells were transplanted into secondary recipients (4 secondary recipients per primary recipient). All secondary recipients were long-term multilineage reconstituted by donor cells (data not shown). These data confirm the self-renewal potential of individual CD150+CD48–Sca-1+lineage–Mac-1+ fetal liver HSCs.
Fetal liver HSCs are highly enriched in the CD150+CD48– cell population
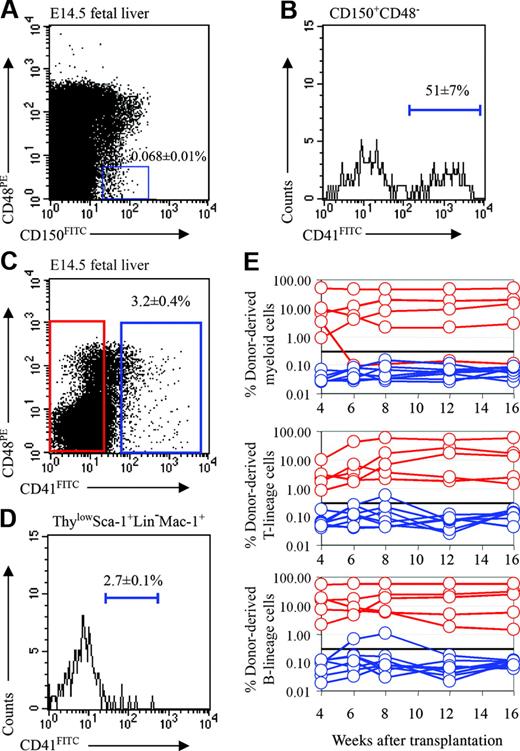
Beyond improving purity, another advantage of the SLAM family markers is their ability to simplify the purification of adult HSCs. CD150+CD48– cells represent only 0.008% of adult bone marrow, and 1 of every 4.8 cells from this population gives long-term multilineage reconstitution upon transplantation into irradiated mice.11 Similarly, CD150+CD48– cells represented only 0.068% ± 0.01% of fetal liver cells (Figure 4A). In adult bone marrow, the purity of HSCs in this population could be further enhanced by excluding megakaryocytes using CD41,11 the platelet GPIIb integrin that is expressed throughout the megakaryocyte lineage.27-30 In adult bone marrow, CD150+CD48–CD41– cells represent only 0.003% of cells, and 45% of the single cells from this population give long-term multilineage reconstitution in irradiated mice.11 We found that 51% ± 7% of fetal liver CD150+CD48– cells expressed CD41 (Figure 4B) and that CD150+CD48–CD41– cells accounted for 0.028% ± 0.01% of fetal liver cells. This raised the possibility that fetal liver HSCs might be highly enriched within this cell population.
Fetal liver HSCs are CD150+. (A) Two-and-a-half percent of fetal liver cells expressed CD150. (B) CD150 expression was heterogeneous within the Thylow Sca-1+lineage–Mac-1+ HSC population. For this analysis, CD150, Thy-1, Sca-1, lineage marker, and Mac-1 staining were detected in the FITC, PE-Cy5, APC-Cy7 (PR), PE, and APC channels, respectively. (C) Recipient mice received transplants of 1250 CD150+ fetal liver cells (blue lines) or 48 750 CD150– fetal liver cells (red lines). All multilineage reconstituting activity was contained within the CD150+ cell fraction (10 of 10 mice) not within the CD150– cell fraction (0 of 10 mice). The CD150– cell fraction showed transient B- and T-cell reconstitution (4 of 10 mice) or transient B-lineage reconstitution (3 of 10 mice), but no detectable myeloid lineage reconstitution.
Fetal liver HSCs are CD150+. (A) Two-and-a-half percent of fetal liver cells expressed CD150. (B) CD150 expression was heterogeneous within the Thylow Sca-1+lineage–Mac-1+ HSC population. For this analysis, CD150, Thy-1, Sca-1, lineage marker, and Mac-1 staining were detected in the FITC, PE-Cy5, APC-Cy7 (PR), PE, and APC channels, respectively. (C) Recipient mice received transplants of 1250 CD150+ fetal liver cells (blue lines) or 48 750 CD150– fetal liver cells (red lines). All multilineage reconstituting activity was contained within the CD150+ cell fraction (10 of 10 mice) not within the CD150– cell fraction (0 of 10 mice). The CD150– cell fraction showed transient B- and T-cell reconstitution (4 of 10 mice) or transient B-lineage reconstitution (3 of 10 mice), but no detectable myeloid lineage reconstitution.
Before testing this directly, we examined whether fetal liver HSCs were CD41+ or CD41–. Only 3.2% of E14.5 fetal liver cells were CD41+ (Figure 4C), and only rare CD41+ cells were contained within the ThylowSca-1+lineage–Mac-1+ cell population (Figure 4D). We nonetheless performed competitive reconstitution assays in which CD41+ or CD41– donor fetal liver cells were transplanted into lethally irradiated recipient mice along with a radioprotective dose of 200 000 recipient-type whole bone marrow cells (Figure 4E). Most of the recipients (4 of 5) that received CD41– cells were long-term multilineage reconstituted, and no recipients (0 of 8) that received CD41+ cells were long-term multilineage reconstituted. These data indicate that most or all of the HSC activity is in the CD41– fraction of fetal liver cells.
Fetal liver HSCs are CD41–. (A) CD150+CD48– cells accounted for 0.068% ± 0.01% of E14.5 fetal liver cells (note that these gates used for the analysis of HSC frequency were somewhat tighter than those used in Figure 1D for the analysis of colony-forming progenitors), and (B) 51% ± 7% of the cells in this population were CD41+. In the analysis shown in panel B, CD41, CD48, and CD150 staining were detected in the FITC, PE, and APC channels, respectively. (C) Three-and-one-fifth percent of fetal liver cells expressed CD41. (D) CD41 expression was detected on only 2.7% of cells within the ThylowSca-1+lineage–Mac-1+ HSC population. In this analysis CD41, Thy-1, Sca-1, lineage marker, and Mac-1 staining were detected in the FITC, PE-Cy5, APC-Cy7 (PR), PE, and APC channels, respectively. (E) Irradiated recipient mice received transplants of 1620 CD41+ fetal liver cells (blue lines) or 48 380 CD41– fetal liver cells (red lines). Most mice that received transplants of CD41– cells became long-term multilineage reconstituted (4 of 5; the other mouse was transiently multilineage reconstituted), but none of the mice that received transplants of CD41+ cells showed convincing reconstitution (0 of 8 mice).
Fetal liver HSCs are CD41–. (A) CD150+CD48– cells accounted for 0.068% ± 0.01% of E14.5 fetal liver cells (note that these gates used for the analysis of HSC frequency were somewhat tighter than those used in Figure 1D for the analysis of colony-forming progenitors), and (B) 51% ± 7% of the cells in this population were CD41+. In the analysis shown in panel B, CD41, CD48, and CD150 staining were detected in the FITC, PE, and APC channels, respectively. (C) Three-and-one-fifth percent of fetal liver cells expressed CD41. (D) CD41 expression was detected on only 2.7% of cells within the ThylowSca-1+lineage–Mac-1+ HSC population. In this analysis CD41, Thy-1, Sca-1, lineage marker, and Mac-1 staining were detected in the FITC, PE-Cy5, APC-Cy7 (PR), PE, and APC channels, respectively. (E) Irradiated recipient mice received transplants of 1620 CD41+ fetal liver cells (blue lines) or 48 380 CD41– fetal liver cells (red lines). Most mice that received transplants of CD41– cells became long-term multilineage reconstituted (4 of 5; the other mouse was transiently multilineage reconstituted), but none of the mice that received transplants of CD41+ cells showed convincing reconstitution (0 of 8 mice).
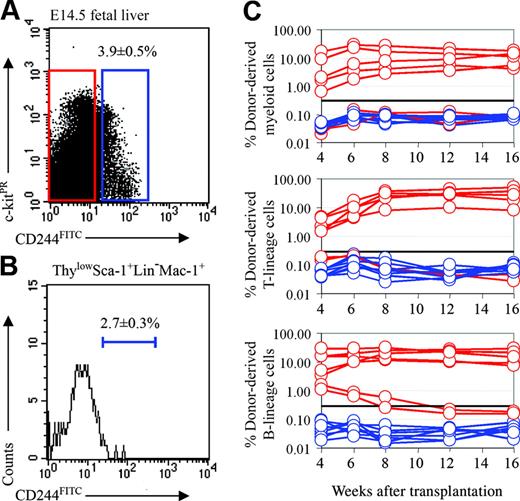
Fetal liver HSCs are CD244–. (A) Three-and-nine-tenths percent of fetal liver cells expressed CD244. (B) CD244 expression was detected in only 2.7% of ThylowSca-1+lineage–Mac-1+ cells. CD244, Thy-1, Sca-1, lineage marker, and Mac-1 staining were detected in the FITC, PE-Cy5, APC-Cy7 (PR), PE, and APC channels, respectively. (C) Irradiated recipient mice received transplants of 1950 CD244+ fetal liver cells (blue lines) or 48 050 CD244– fetal liver cells (red lines). All long-term multilineage reconstituting activity was contained within the CD244– cell fraction (5 of 7; the other 2 mice were reconstituted only in the B-cell lineage). None of the mice that received transplants of CD244+ cells (0 of 8) showed any donor cell reconstitution.
Fetal liver HSCs are CD244–. (A) Three-and-nine-tenths percent of fetal liver cells expressed CD244. (B) CD244 expression was detected in only 2.7% of ThylowSca-1+lineage–Mac-1+ cells. CD244, Thy-1, Sca-1, lineage marker, and Mac-1 staining were detected in the FITC, PE-Cy5, APC-Cy7 (PR), PE, and APC channels, respectively. (C) Irradiated recipient mice received transplants of 1950 CD244+ fetal liver cells (blue lines) or 48 050 CD244– fetal liver cells (red lines). All long-term multilineage reconstituting activity was contained within the CD244– cell fraction (5 of 7; the other 2 mice were reconstituted only in the B-cell lineage). None of the mice that received transplants of CD244+ cells (0 of 8) showed any donor cell reconstitution.
To test the purity of HSCs in this population, we transplanted single fetal liver CD150+CD48–CD41– cells into irradiated recipient mice along with 200 000 bone marrow cells (Table 2). One (18%) of every 5.7 CD150+CD48–CD41– cells gave long-term multilineage reconstitution. Thus, these markers make it possible to highly enrich fetal liver HSCs using a simple combination of markers, though they do not provide quite as high purity as in adult bone marrow or in the more complex fetal CD150+CD48–Sca-1+ lineage–Mac-1+ population.
CD244 is not expressed by fetal liver HSCs
In adult bone marrow, CD244 is expressed by some transiently reconstituting multipotent progenitors but not by HSCs.11 To test whether this aspect of the SLAM family expression pattern also is conserved between fetal liver and adult bone marrow, we examined the expression of CD244 in the fetal liver. CD244+ cells represented only 3.9% ± 0.5% of cells in the fetal liver (Figure 5A), and only 2.7% ± 0.3% of cells in the ThylowSca-1+lineage–Mac-1+ HSC population (Figure 5B). To test whether fetal liver HSCs were CD244+ or CD244–, we performed competitive reconstitution assays in which CD244+ or CD244– donor cells were transplanted into lethally irradiated recipient mice along with a radioprotective dose of 200 000 recipient-type whole bone marrow cells (Figure 5C). Of 7 recipients that received CD244– cells, 5 were long-term multilineage reconstituted, but no recipients (0 of 8) that received CD244+ cells showed any reconstitution. These data indicate that most or all of the HSC activity in fetal liver is in the CD244– fraction, just as previously observed in bone marrow. However, whereas some transiently reconstituting multipotent progenitors were CD244+ in adult bone marrow, there was no multipotent progenitor activity among CD244+ fetal liver cells.
SLAM family markers are not general markers of stem cells in other fetal or adult tissues
To test whether stem cells in other tissues also were marked by CD150, CD48, and/or CD244 expression, we examined CD150, CD244, and CD48 expression in the fetal and adult central (CNS) and peripheral (PNS) nervous systems. CD150, CD48, and CD244 rarely stained CD45– cells in any of these tissues, including the E14.5 telencephalon, the E14.5 gut (including both gut epithelium and developing enteric nervous system; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), the adult lateral ventricle subventricular zone, and the adult outer gut wall (including muscle layers and enteric nervous system; Figure S2). This indicates that CD150, CD244, and CD48 are rarely (if at all) expressed in the fetal or adult nervous system or the fetal gut, outside of the CD45+ hematopoietic cells in these tissues.
To functionally test whether neural stem cells in the CNS or PNS express SLAM family receptors, we sorted SLAM receptor positive or negative cells from the E14.5 gut, the E14.5 telencephalon, and the adult lateral ventricle subventricular zone into culture.31-33 CD150–, CD48–, and CD244– cells consistently formed multilineage neural stem-cell colonies in culture, but CD150+, CD48+, and CD244+ cells rarely formed neural stem-cell colonies (Figures S1-S2). This indicates that CD150, CD244, and CD48 are not general markers for stem cells outside of the hematopoietic system.
Discussion
In this study we have demonstrated that the SLAM family receptors CD150, CD244, and CD48 are differentially expressed among fetal hematopoietic progenitors in a way that is similar to that observed in adult bone marrow.11,24 Just as in adult bone marrow, CD150 was expressed on only a few percent of fetal liver cells, and yet most or all HSCs were CD150+ (Figure 3, Table 2). Just as in adult bone marrow, most colony-forming progenitors in the fetal liver were CD48+, with almost 90% of all colony-forming progenitors falling within the CD48+CD244–CD150– and CD48+CD244+CD150– fractions, which accounted for fewer than 30% of fetal liver cells (Figure 1D). CD48+ cells also included restricted B-lineage progenitors that gave transient B-lineage reconstitution in some irradiated mice (Figure 1C), just as observed in adult bone marrow. The only differences in SLAM receptor expression that were evident between fetal liver and adult bone marrow were that while CD244+ cells in adult bone marrow included transiently reconstituting multipotent progenitors and most colony-forming progenitors, CD244+ fetal liver cells did not give any detectable reconstituting activity and included a minority of colony-forming progenitors (Figures 1D, 5C). Nonetheless, at least some transiently reconstituting multipotent progenitors in fetal liver were CD150–, as observed in adult bone marrow. These results indicate that SLAM family receptor expression patterns are similar but not identical in adult and fetal liver hematopoiesis.
The use of SLAM family markers enhanced the purification of fetal liver HSCs beyond what had been possible previously. Approximately 1 of every 8 ThylowSca-1+lineage–Mac-1+ fetal liver cells6 or Tie-2+Sca-1+lineage–AA4.1+ fetal liver cells7 give long-term multilineage reconstitution upon transplantation into irradiated mice. We found that ThylowSca-1+lineage–Mac-1+ fetal liver cells were heterogeneous, with 51% of these cells expressing CD48 (Figure 1B) and lacking reconstituting activity in irradiated mice (Table 1). Similarly, approximately 60% of ThylowSca-1+ lineage–Mac-1+ fetal liver cells were CD150– (Figure 3B) transiently reconstituting progenitors (Table 2). By selecting the CD150+CD48– subset of Sca-1+lineage–Mac-1+ fetal liver cells, we were able to significantly improve the purity of this population, with 37% of the single cells in this population giving long-term multilineage reconstitution in irradiated mice (11 of 30 mice in 2 experiments; 1 in 2.7; Table 2). Moreover, even a simplified combination of markers gave highly enriched fetal liver HSCs, with 1 (18%) of every 5.7 CD150+CD48–CD41– cells giving long-term multilineage reconstitution in irradiated mice. This makes it possible to highly enrich fetal liver HSCs based on a 2-color stain. Together, these data indicate that the use of SLAM family members can substantially increase the purity of fetal liver HSCs and that fetal liver HSCs are capable of reconstituting irradiated adult mice with very high efficiency, just as previously observed with adult HSCs.8-11
The similar expression patterns of SLAM family receptors in both fetal and adult hematopoiesis emphasize the robustness with which SLAM family markers can be used to purify HSCs and to distinguish different types of hematopoietic progenitors. HSCs can be highly enriched within either the CD150+CD48–CD244–CD41– fraction (using a 2-color stain) or in the CD150+CD48–Sca-1+ lineage–Mac-1+ fraction (using a 5-color stain) of fetal liver (this study), young adult bone marrow,11 cytokine mobilized spleen,17,24 old adult bone marrow,24 and reconstituted bone marrow.24 These data indicate that beyond being tools that enhance the precision with which HSCs can be studied, these SLAM family receptors may be functionally important in regulating hematopoiesis. CD150 is a self-ligand,34 raising the possibility that it could regulate interactions among HSCs or among HSCs and activated lymphocytes, which can also express CD150. Studies of CD150-deficient mice so far have not revealed a hematopoietic phenotype,11 though additional studies are ongoing to determine whether CD150 is critical for regulating HSCs in certain contexts beyond steady-state hematopoiesis. Similarly, CD244 binds CD48,34 raising the possibility that these SLAM family members might mediate the interaction of progenitors at different levels of the hematopoiesis hierarchy. Additional studies in CD244-deficient and CD48-deficient mice will be required to test this.
Rare CD150+CD48–Sca-1+lineage–Mac-1+ cells also were found in the E9.5 to E11.5 yolk sac, whole embryo, and AGM, raising the possibility that these markers might enhance the identification of HSCs or their precursors prior to the onset of definitive hematopoiesis (data not shown). However, in contrast to their highly restricted expression after the onset of definitive hematopoiesis, CD150+CD48–CD244– cells were surprisingly abundant in the E9.5 whole embryo (data not shown). This indicates that these markers are not restricted to HSCs or even hematopoietic cells during early embryonic development, though additional experiments will be required to determine the identities of cells that express SLAM family markers before E11.5. Nonetheless, during fetal and adult stages, CD150, CD244, and CD48 did not appear to be expressed on stem cells or significant numbers of nonhematopoietic cells in other tissues as neural stem cells and other cells from the fetal and adult CNS, fetal and adult enteric nervous system, and fetal gut did not appear to express any of these receptors (Figures S1-S2). These results indicate that these SLAM family receptors are not general markers for stem cells outside of the hematopoietic system.
Irrespective of the functions of SLAM family receptors in hematopoiesis, these results support previous findings in indicating that these receptors are differentially expressed among hematopoietic progenitors at different stages of the hematopoiesis hierarchy. These patterns are similar in fetal and adult hematopoiesis. The clear differential expression makes it possible to dramatically enhance the purification of fetal liver HSCs as CD150+CD48– cells. These results demonstrate that fetal liver HSCs can highly efficiently reconstitute irradiated adult mice, with at least 37% of single CD150+CD48–Sca-1+lineage–Mac-1+ cells giving long-term multilineage reconstitution. These results suggest that HSCs isolated from diverse contexts of fetal and adult hematopoiesis have the ability to reconstitute irradiated mice with high efficiency.
Prepublished online as Blood First Edition Paper, March 28, 2006; DOI 10.1182/blood-2005-10-4135.
Supported by the Howard Hughes Medical Institute and the United States Army Research Laboratory/Office (grant DAAD19-03-1-0168). Partially supported by the University of Michigan (UM)–Comprehensive Cancer National Institutes of Health (NIH) (grant CA46592), the UM–Multipurpose Arthritis Center NIH (grant AR20557), the Rheumatic Core Disease Center (grant 1 P30 AR48310), a postdoctoral fellowship from University of Michigan Cancer Biology Training Program (I.K.), a predoctoral fellowship from the UM Institute of Gerontology (ö.H.Y.), and a Medical Scientist Training Program Fellowship (M.J.K.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Dan Cua at DNAX for providing the 26D12 antibody against CD150. Thanks to David Adams and Martin White for flow cytometry and to Elizabeth Smith (University of Michigan Hybridoma Core Facility, Ann Arbor) for antibody production.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal