Studies of the human immune system have been limited by the lack of an appropriate in vivo model. For this reason, efforts have been made to develop murine models with a functional human immune system. We report here that cotransplantation of human fetal thymus/liver tissues and CD34+ hematopoietic stem/progenitor cells led to the development of sustained human hematopoiesis and a functional human immune system in immunodeficient NOD/SCID mice. The humanized mice showed systemic repopulation with a comprehensive array of human lymphohematopoietic cells, including T cells, B cells, and dendritic cells, and the formation of secondary lymphoid organs. Furthermore, these mice produce high levels of human IgM and IgG antibodies and mediate strong immune responses in vivo as demonstrated by skin xenograft rejection. Thus, the humanized NOD/SCID mice described in this paper provide a powerful model system to study human immune function.
Introduction
Human hematopoietic tissue and cell transplantation into immunodeficient mice, such as mice with the severe combined immunodeficiency (SCID)1,2 mutation and their derivatives, including NOD/SCID,3 NOD/SCID/β2mnull,4 and NOD/SCID/γcnull mice,5 has been widely used to study the function of human immune cells. However, only a few studies have shown the ability to achieve systemic in vivo immune responses in humanized mice.6 In general, immunodeficient mice that received a transplant of human hematopoietic stem cells (HSCs) have poor human T-cell development. Although some human T-cell reconstitution was seen in NOD/SCID/γcnull mice after human umbilical cord blood (UCB) stem-cell transplantation, human thymopoiesis in the recipient thymus appeared inefficient, and the mouse thymus remained underdeveloped in these mice.5 Recently, Traggiai et al6 reported that intrahepatic injection of human CD34+ UCB cells into newborn Rag2–/–γc–/– mice results in improved human T-cell development in the mouse thymus. An important advancement in this model compared with UCB transplantation in adult mice was the ability of grafted mice to mediate in vivo antiviral immune responses.6 However, a long period of time (16 weeks) was required to achieve significant peripheral human T-cell repopulation in these mice, and the levels of human T cells in the spleen of long-term surviving mice were relatively low. Furthermore, the MHC restriction and tolerance status of human T cells that were selected in the mouse thymus in these mice still remains undefined.
A commonly used mouse model for the study of human thymopoiesis and T-cell development has been the human-SCID mouse model introduced by McCune et al,1,7 in which SCID mice received a transplant of human fetal thymus (Thy) and fetal liver (Liv) tissues. In this model, human T cells develop in the autologous human thymus graft. This model has been successfully applied to studies of the biology of human thymopoiesis7 and human immunodeficiency virus (HIV) infection of human T cells.8,9 However, the grafted mice have not been shown to be capable of mediating normal immune responses in vivo. In this study, we demonstrate that the addition of human CD34+ cell infusion to fetal human Thy/Liv transplantation permits the reconstitution of a functional human immune system in immuno-deficient mice. While human thymopoiesis was achieved in NOD/SCID mice grafted with human Thy/Liv alone, and human T cells from these mice were functional when tested by in vitro assays, these mice failed to mediate efficient immune responses in vivo. Strong in vivo immune responses leading to rejection of skin xenografts were observed in mice that received a graft of Thy/Liv only when CD34+ fetal liver cells (FLCs) were simultaneously transplanted. Furthermore, mice that received Thy/Liv/CD34+ FLCs showed repopulation with multilineage human hematopoietic cells, including T cells, B cells, and dendritic cells (DCs) in secondary lymphoid tissues, whereas almost all human cells detected in mice that received only Thy/Liv were CD3+ T cells. Thus, combined transplantation of human fetal Thy/Liv and intravenous injection of CD34+ cells provide a useful in vivo model for the study of the human immune system.
Materials and methods
Animals and human fetal tissues
Immunodeficient nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice were housed in a specific pathogen-free microisolator environment and used at 6 to 10 weeks of age. Human fetal thymus and liver tissues of gestational age of 17 to 20 weeks were obtained from Advanced Bioscience Resource (Alameda, CA). Inbred Massachusetts General Hospital miniature swine (kindly provided by David H. Sachs)10 were used as porcine skin donors. Protocols involving the use of human tissues and animals were approved by the Massachusetts General Hospital Human Research Committee and Subcommittee on Research Animal Care, and all of the experiments were performed in accordance with the protocols.
Human tissue implantation
Mice were conditioned with sublethal (2-3 Gy) whole-body irradiation. Human fetal Thy and Liv fragments measuring about 1 mm3 were implanted under the recipient kidney capsule within 3 days after irradiation. Some mice also received CD34+ FLCs (1-5 × 105/mouse, intravenously) purified from the same donor on the day of human Thy/Liv transplantation. CD34+ FLCs were isolated by the magnetic-activated cell sorter (MACS) separation system using anti-CD34 microbeads (Miltenyi Biotec, Auburn, CA). Levels of human hematopoietic cells in the mice that underwent transplantation were determined by multicolor flow cytometric (FCM) analysis using various combinations of the following mAbs: anti–HLA class I (W6/32; Leinco Technologies, St Louis, MO); anti–HLA-DR; anti–human CD3, CD4, CD8, CD11c, CD19, CD20, CD45, and CD45RA; anti–mouse CD45; and isotype control mAbs (all purchased from BD PharMingen, San Diego, CA). FACS analysis was performed on a FACScalibur (Becton Dickinson, Mountain View, CA). Dead cells were excluded from the analysis by gating out lower forward scatter and high propidium iodide–retaining cells.
Mixed lymphocyte reaction assay
Briefly, triplicate wells containing 4 × 105 responders and 4 × 105 irradiated (30 Gy) stimulators were incubated in AIM-V medium containing 10% human AB serum (Sigma, St Louis, MO) at 37°C in 5% CO2. Cultures were pulsed with [3H]-TdR on day 3 and harvested 18 hours later. Data are expressed as stimulation index (cpm of stimulated culture/cpm of unstimulated culture). Unstimulated control cultures were the same responder cells incubated with medium or irradiated autologous human fetal liver cells that were cryopreserved at the time of human Thy/Liv implantation.
Skin grafting
Split thickness (2.2 mm) porcine skin samples were grafted on the lateral thoracic wall 6 weeks after human tissue transplantation. Skin grafts were evaluated daily from day 7 onward up to 4 weeks, and once every third day thereafter. Grafts were defined as rejected when less than 10% of the graft remained viable.
Histology
Tissues were cryosectioned (4 μm) and fixed with cold acetone (for immunohistochemical and fluorescent staining), or fixed in 10% buffered formalin and embedded in paraffin (for hematoxylin and eosin [H&E] staining). Two-color immunohistochemistry was performed on spleen and lymph node sections with the following primary antibodies: polyclonal rabbit anti–human CD3 antibody (DAKO, Glostrup, Denmark), mouse anti–human CD20 mAb (L26; DAKO), mouse anti–human CD11c mAb (KB90; DAKO), mouse anti–human CD123 mAb (6H6; eBioscience, San Diego, CA), and rat anti–human HLA-DR mAb (YD1/63.4.10; Serotec, Oxford, United Kingdom). Dual immunohistochemical staining was conducted by the combination of immunoalkaline phosphatase technique (blue color) and avidin-biotin peroxidase complex (ABC) technique with H2O2 containing DAB (brown color). Slides were observed using an Olympus BX40 light microscope (Olympus, Melville, NY) with 20 × /0.5 numeric aperture (NA) and 40 × /0.75 NA objectives, and were photographed using a Hitachi HV-C20 color camera (Hitachi, Nashua, NH). Human T-cell infiltration in porcine skin grafts was determined by immunofluorescence staining using FITC-conjugated anti–human CD3 mAb (UCHT1; BD PharMingen). After being washed and mounted, slides were viewed under a Nikon Eclipse TE2000-U fluorescent microscope using a 20 × /0.5 NA objective, and were photographed by a DS-U1 digital camera (Nikon, Melville, NY) using Adobe Photoshop Elements software version 3.0 (Adobe Systems, San Jose, CA).
ELISA for human IgM and IgG detection
Human IgM and IgG antibodies were detected using human IgM and human IgG enzyme-linked immunosorbent assay (ELISA) quantitation kit as directed by the manufacturer (Bethyl Laboratories, Montgomery, TX).
Statistical analysis
Statistical analysis of survival data was performed with the log-rank test, and the level of significant differences in group means was determined by Student t test for parametric data set, or by Mann-Whitney test for nonparametric data set. All statistical analysis was performed using Prism 4 (GraphPad Software, San Diego, CA). A P value of less than .05 was considered significant in all types of analyses.
Results
CD34+ FLC transplantation (intravenous) leads to multilineage human hematopoietic repopulation and improves human T-cell development in NOD/SCID mice that received a graft of human Thy/Liv
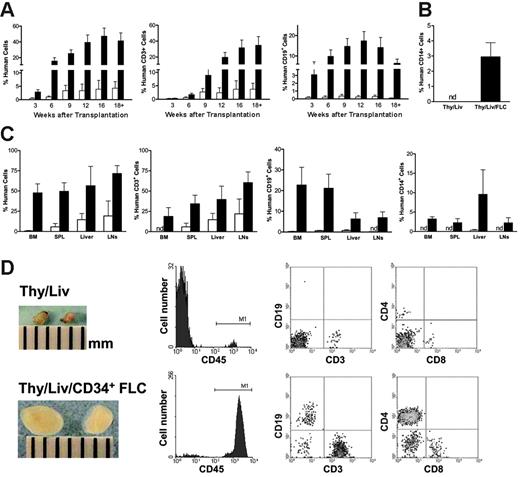
Previous studies have shown that human fetal Thy/Liv grafting under the kidney capsule of NOD/SCID mice can lead to human thymopoiesis and T-cell development in vivo.7,11 We have now explored the possibility of achieving complete human hematopoietic repopulation in NOD/SCID mice by combined transplantation of human Thy/Liv and CD34+ FLCs. CD34+ FLCs were prepared from the liver of the same fetus and injected intravenously into recipient mice immediately after Thy/Liv implantation. As shown in Figure 1, the addition of CD34+ FLC transplantation significantly improved human hematopoietic repopulation in NOD/SCID mice that received a graft of Thy/Liv. Although mice receiving human Thy/Liv alone showed repopulation with human cells in peripheral blood, the majority of these human cells were CD3+ T cells (Figure 1A-B). In contrast, combined transplantation of CD34+ FLCs (intravenous) and Thy/Liv led to sustained repopulation with multilineage human hematopoietic cells, including CD3+ T cells, CD19+ B cells, as well as CD14+ monocytes/macrophages (Figure 1A-B). The significantly greater T-cell repopulation in mice that received CD34+ FLCs suggests that CD34+ FLC injection also improves human thymopoiesis and T-cell development and/or survival in NOD/SCID mice that received a graft of Thy/Liv.
Human hematopoietic-cell repopulation in other tissues was analyzed when mice were killed 18 to 25 weeks after transplantation. Mice that received Thy/Liv/CD34+ FLCs showed multilineage human hematopoietic-cell repopulation in the BM, spleen, liver, and lymph nodes, whereas almost all human cells detected in mice that received Thy/Liv alone were CD3+ T cells (Figure 1C-D). The levels of human CD3+ T (including both CD4+ and CD8+) cells in these tissues were also significantly increased in mice that underwent combined Thy/Liv and CD34+ FLC transplantation. Furthermore, the lymph nodes, including cervical, axillary, brachial, renal, mesenteric, and inguinal nodes, in mice that received Thy/Liv/CD34+ FLCs were significantly enlarged to a similar size as lymph nodes found in immunocompetent mice (Figure 1D). The majority (90% ± 5%) of lymph node cells were human cells as identified by human CD45 expression, including CD3+ (both CD4+ and CD8+) T cells, CD19+ B cells (Figure 1C-D), and DCs (Figure 2). In contrast, lymph nodes from mice that received Thy/Liv without CD34+ FLCs remained small and frail, similar to those of normal NOD/SCID mice. Only low levels of human cells were detected in these lymph nodes, and most of them were human T cells (human CD45+ and CD3+ cells were 2.2% ± 1.3% and 2% ± 1.2%, respectively).
Transplantation of CD34+ FLCs improves multilineage human hematopoiesis in NOD/SCID mice that received a graft of human fetal Thy/Liv. NOD/SCID mice that received Thy/Liv only (□,n = 8) or Thy/Liv/CD34+ FLCs (▴,n = 13) were bled to measure human chimerism in the peripheral blood mononuclear cells (PBMCs) at the indicated time points; all mice were killed between 18 to 25 weeks after human tissue/cell transplantation for analysis of human-cell repopulation in bone marrow (BM), spleen (SPL), liver, and lymph nodes (LNs). (A-C) Data are mean ± SEMs, and the differences in mean values at the time points later than 6 weeks are statistically significant. (A) Kinetics of human CD45+, CD3+, and CD19+ cell levels in the PBMCs. (B) Levels of human CD14+ monocytes/macrophages in the PBMCs at death between 18 and 25 weeks after human tissue/cells transplantation. (C) Levels of various lineages of human hematopoietic cells in BM, SPL, liver, and LNs at death between 18 and 25 weeks after human tissue/cell transplantation. nd indicates not detected. (D) Macroscopic and FCM analyses of representative mesenteric lymph nodes taken from NOD/SCID mice that received Thy/Liv (top panel) or Thy/Liv/CD34+ FLCs (bottom panel) at week 20. For FCM analysis, single-cell suspensions were stained for the markers of human hematopoietic cells (CD45), T cells (CD3, CD4, and CD8), and B cells (CD19).
Transplantation of CD34+ FLCs improves multilineage human hematopoiesis in NOD/SCID mice that received a graft of human fetal Thy/Liv. NOD/SCID mice that received Thy/Liv only (□,n = 8) or Thy/Liv/CD34+ FLCs (▴,n = 13) were bled to measure human chimerism in the peripheral blood mononuclear cells (PBMCs) at the indicated time points; all mice were killed between 18 to 25 weeks after human tissue/cell transplantation for analysis of human-cell repopulation in bone marrow (BM), spleen (SPL), liver, and lymph nodes (LNs). (A-C) Data are mean ± SEMs, and the differences in mean values at the time points later than 6 weeks are statistically significant. (A) Kinetics of human CD45+, CD3+, and CD19+ cell levels in the PBMCs. (B) Levels of human CD14+ monocytes/macrophages in the PBMCs at death between 18 and 25 weeks after human tissue/cells transplantation. (C) Levels of various lineages of human hematopoietic cells in BM, SPL, liver, and LNs at death between 18 and 25 weeks after human tissue/cell transplantation. nd indicates not detected. (D) Macroscopic and FCM analyses of representative mesenteric lymph nodes taken from NOD/SCID mice that received Thy/Liv (top panel) or Thy/Liv/CD34+ FLCs (bottom panel) at week 20. For FCM analysis, single-cell suspensions were stained for the markers of human hematopoietic cells (CD45), T cells (CD3, CD4, and CD8), and B cells (CD19).
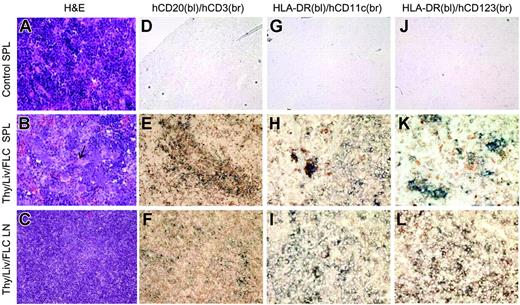
Immune-cell population and structural reconstitution of spleen and lymph nodes in NOD/SCID mice that received human Thy/Liv/CD34+ FLCs. Spleens and lymph nodes were harvested from control NOD/SCID mice (n = 6) and NOD/SCID mice that received human Thy/Liv/CD34+ FLCs at death between 18 and 25 weeks after human tissue/cell transplantation (n = 5), and assessed by H&E and immunohistochemical staining. (A-C) H&E staining of representative spleen samples from control NOD/SCID mice (A) and NOD/SCID mice that received Thy/Liv/CD34+ FLCs (B, arrow indicates a central artery), and mesenteric lymph node (C) from NOD/SCID mice that received Thy/Liv/CD34+ FLCs (original magnification, × 200). (D-L) Immunohistochemical staining of representative spleen sections from control NOD/SCID mice (D,G,J) and NOD/SCID mice that received a graft of Thy/Liv/CD34+ FLCs (E,H,K), and mesenteric lymph node sections from NOD/SCID mice that received a graft of Thy/Liv/CD34+ FLCs (F,I,L). Shown are results of 2-color immunohistochemistry with anti–human CD20 (blue) and anti–human CD3 (brown) (D-F; original magnification, × 200), anti–HLA-DR (blue) and anti–human CD11c (brown) (G-I; original magnification, × 400), or anti–HLA-DR (blue) and anti–human CD123 (brown) (J-L; original magnification, × 400). bl indicates blue; br, brown.
Immune-cell population and structural reconstitution of spleen and lymph nodes in NOD/SCID mice that received human Thy/Liv/CD34+ FLCs. Spleens and lymph nodes were harvested from control NOD/SCID mice (n = 6) and NOD/SCID mice that received human Thy/Liv/CD34+ FLCs at death between 18 and 25 weeks after human tissue/cell transplantation (n = 5), and assessed by H&E and immunohistochemical staining. (A-C) H&E staining of representative spleen samples from control NOD/SCID mice (A) and NOD/SCID mice that received Thy/Liv/CD34+ FLCs (B, arrow indicates a central artery), and mesenteric lymph node (C) from NOD/SCID mice that received Thy/Liv/CD34+ FLCs (original magnification, × 200). (D-L) Immunohistochemical staining of representative spleen sections from control NOD/SCID mice (D,G,J) and NOD/SCID mice that received a graft of Thy/Liv/CD34+ FLCs (E,H,K), and mesenteric lymph node sections from NOD/SCID mice that received a graft of Thy/Liv/CD34+ FLCs (F,I,L). Shown are results of 2-color immunohistochemistry with anti–human CD20 (blue) and anti–human CD3 (brown) (D-F; original magnification, × 200), anti–HLA-DR (blue) and anti–human CD11c (brown) (G-I; original magnification, × 400), or anti–HLA-DR (blue) and anti–human CD123 (brown) (J-L; original magnification, × 400). bl indicates blue; br, brown.
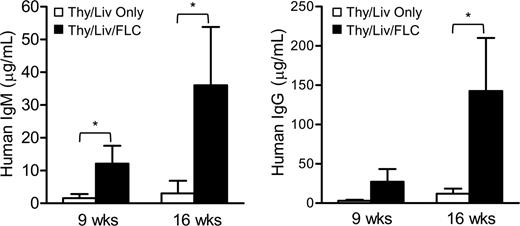
Transplantation of CD34+ FLCs improves the production of human immunoglobulin in NOD/SCID mice that received a graft of human fetal Thy/Liv. At the indicated time points after human tissue/cell transplantation, serum was collected and human IgM (left) and IgG (right) were detected by ELISA. Open bars represent NOD/SCID mice that received human fetal Thy/Liv only (n = 4), and closed bars represent mice that received Thy/Liv and CD34+ FLCs (n = 6). Data are mean ± SEMs. *P < .05.
Transplantation of CD34+ FLCs improves the production of human immunoglobulin in NOD/SCID mice that received a graft of human fetal Thy/Liv. At the indicated time points after human tissue/cell transplantation, serum was collected and human IgM (left) and IgG (right) were detected by ELISA. Open bars represent NOD/SCID mice that received human fetal Thy/Liv only (n = 4), and closed bars represent mice that received Thy/Liv and CD34+ FLCs (n = 6). Data are mean ± SEMs. *P < .05.
Histologic analysis of secondary lymphoid tissues from NOD/SCID mice that received human fetal Thy/Liv/CD34+ FLCs
We next analyzed human T-cell, B-cell, and DC repopulation in secondary lymphoid tissues by histology. H&E staining showed numerous erythroblasts (which are stained dark blue and morphologically resemble lymphocytes) and no follicular structure in the spleens of the control NOD/SCID mice (Figure 2A). In the spleens of mice that received human Thy/Liv/CD34+ FLCs, while typical splenic structure was not identified, erythroblasts were replaced by lymphocytes, and white pulplike structures characterized by the accumulation of lymphocytes around the central artery were observed (Figure 2B). Dual immunohistochemical staining revealed the presence of human T and B cells in the spleen (Figure 2E) and lymph nodes (Figure 2F) from mice that received human Thy/Liv/CD34+ FLCs. Human T cells and B cells populated the spleen in a segregated manner; T cells gathered around the central artery, and B cells formed clusters adjacent to the T cells (Figure 2E). However, follicular structure was not detected in the lymph nodes from these mice (Figure 2C,F). Furthermore, dual immunohistochemical staining for HLA-DR and human CD11c or CD123 demonstrated the presence of both myeloid (HLA-DR+CD11c+) and plasmacytoid (HLA-DR+CD123+) DCs in the spleen and lymph nodes from mice that received Thy/Liv/CD34+ FLCs (Figure 2H,K,I,L). The presence of human DCs in the spleen and lymph nodes from mice that received Thy/Liv/CD34+ FLCs, but not those that received only Thy/Liv, was also demonstrated by FCM analysis, in which DCs were identified as lineage negative (Lin–) HLA-DR+ (CD11c+ or CD11c–) (data not shown). These results show that in NOD/SCID mice that received a transplant of human fetal Thy/Liv plus CD34+ FLCs, secondary lymphoid organs were populated with various types of human immune cells necessary for the induction of antigen-specific immune responses, and at least the spleen was populated with these cells in a structurally ordered manner.
The presence of human T cells, B cells, and DCs in the lymph nodes suggests that the adhesion molecules and chemokines essential for the homing of human cells to lymph nodes are unlikely to be species specific. To further address species specificity of adhesion molecules and chemokines as a cause of the failure to establish microstructure in lymph nodes of mice that received a transplant of Thy/Liv/CD34+ FLCs, we performed bone marrow transplantation from immunocompetent NOD mice to NOD/SCID mice. Similar to the lymph nodes from mice that received a transplant of human Thy/Liv/CD34+ FLCs, lymph nodes in NOD/SCID mouse recipients of NOD marrow also did not show an organized microstructure (data not shown). It has been shown that lymphotoxin (LT)–mediated signals provided by fetal liver–derived lymphoid tissue–inducer cells and lymphocytes during organogenesis are essential for the formation of lymphoid organs.12,13 Our data suggest that the lymph node anlagen formed in the absence of lymphocytes in NOD/SCID mice during organogenesis, but that proper signals such as LT-mediated signals from lymphocytes during early lymph development may be critical for lymph nodes to form and maintain a highly organized microstructure.
Enhanced human immunoglobulin production in NOD/SCID mice that received a transplant of human fetal Thy/Liv plus CD34+ FLCs
To determine human B-cell function, we measured serum human IgM and IgG by ELISA assay (Figure 3). Consistent with the level of human B-cell repopulation measured by FCM and immunohistochemical analyses (Figures 1, 2), high levels of human IgM and IgG were detected in sera from mice that received a transplant of Thy/Liv/CD34+ FLCs. The efficient human Ig production and IgG class switching indicate that human B cells developing in these humanized mice were functional. However, there were very low levels of human IgM and IgG, if any, in the serum of the mice that received Thy/Liv alone. Thus, simultaneous CD34+ FLC infusion is required for the development and differentiation of functional human B cells in mice that received a graft of human fetal Thy/Liv.
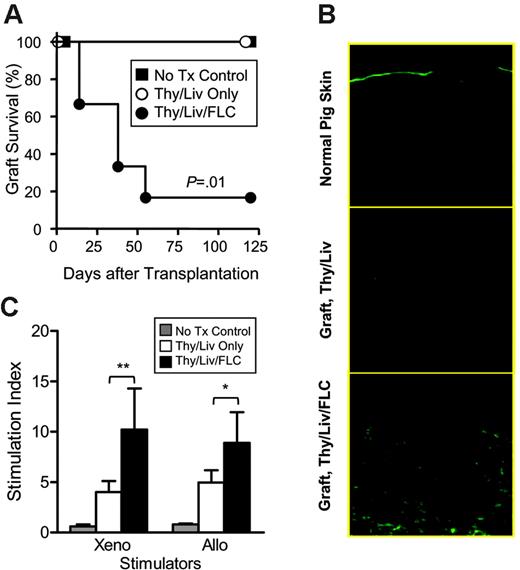
CD34+ FLC infusion improves in vivo functions of human T cells in NOD/SCID mice that received a graft of human fetal Thy/Liv. (A) Skin xenograft survival in NOD/SCID control mice (n = 5), NOD/SCID mice that received human Thy/Liv (n = 5), and NOD/SCID mice that received human Thy/Liv/CD34+ FLCs (n = 6). (B) Fluorescence microscopy showing infiltration by human CD3+ T cells in representative porcine skin grafts from NOD/SCID mice that received a transplant of Thy/Liv and Thy/Liv/CD34+ FLCs. Normal porcine skin was used as staining control. (C) Splenocytes were prepared from NOD/SCID control mice that did not undergo transplantation (n = 5), NOD/SCID mice that received Thy/Liv (n = 5), and NOD/SCID mice that received Thy/Liv/CD34+ FLCs (n = 6) 120 days after skin grafting and analyzed for xenogeneic (antipig) and allogeneic (antihuman) MLR. Data are mean ± SEMs. *P < .05; **P < .01.
CD34+ FLC infusion improves in vivo functions of human T cells in NOD/SCID mice that received a graft of human fetal Thy/Liv. (A) Skin xenograft survival in NOD/SCID control mice (n = 5), NOD/SCID mice that received human Thy/Liv (n = 5), and NOD/SCID mice that received human Thy/Liv/CD34+ FLCs (n = 6). (B) Fluorescence microscopy showing infiltration by human CD3+ T cells in representative porcine skin grafts from NOD/SCID mice that received a transplant of Thy/Liv and Thy/Liv/CD34+ FLCs. Normal porcine skin was used as staining control. (C) Splenocytes were prepared from NOD/SCID control mice that did not undergo transplantation (n = 5), NOD/SCID mice that received Thy/Liv (n = 5), and NOD/SCID mice that received Thy/Liv/CD34+ FLCs (n = 6) 120 days after skin grafting and analyzed for xenogeneic (antipig) and allogeneic (antihuman) MLR. Data are mean ± SEMs. *P < .05; **P < .01.
Improved immune function leading to skin xenograft rejection in NOD/SCID mice that received a transplant of human fetal Thy/Liv plus CD34+ FLCs
Graft rejection requires donor antigen presentation to T cells, T-cell activation and expansion, as well as trafficking of effector cells (eg, activated T cells) to the graft. Thus, graft rejection reflects multiple components of in vivo immune capability. We therefore performed skin xenografting to assess in vivo immune function of the humanized mice. Porcine skin grafting was performed 6 weeks after human tissue/cell transplantation. As shown in Figure 4A, graft rejection was seen in 5 of 6 NOD/SCID mice that received Thy/Liv/CD34+ FLCs, while both the mice that did not undergo transplantation (n = 5) and the mice that received Thy/Liv along (n = 5) kept their grafts long term. Immunohistochemical analysis revealed significantly increased infiltration of human CD3+ T cells in the skin grafts from mice that received Thy/Liv/CD34+ FLCs compared with mice that received only Thy/Liv (Figure 4B). We also measured human T-cell function in vitro by mixed lymphocyte reaction (MLR) assay. In contrast to skin graft rejection, spleen cells from mice that received only Thy/Liv also showed antipig xenogeneic and antihuman allogeneic MLR responses (Figure 4C). The lack of detectable MLR in splenocytes from control NOD/SCID mice that did not undergo transplantation indicates that MLR responses detected in mice that received human Thy/Liv or Thy/Liv/CD34+ FLCs were mediated by human T cells. The lower magnitude of MLR responses for the mice receiving only Thy/Liv than those receiving Thy/Liv plus CD34+ FLCs is presumably due to the smaller number of human T cells in the spleen in the former group. These results indicate that functional human T cells can be generated in both mice that received Thy/Liv and those that received Thy/Liv/CD34+ FLCs, but only the latter model provides a microenvironment suitable for the induction of in vivo human immune responses.
Discussion
In this study, we show that CD34+ FLC infusion can significantly improve human hematopoietic repopulation and lymphopoiesis in NOD/SCID mice that received a graft of human fetal Thy/Liv. NOD/SCID mice that received Thy/Liv plus CD34+ FLCs, but not those that received Thy/Liv alone, showed systemic repopulation with multilineage human lymphohematopoietic cells, including T cells, B cells, and DCs, and were capable of mediating strong immune responses in vivo. It has been reported that postthymic self-recognition is needed to maintain the antigen reactivity of mature T cells and that interruption of T-cell contact with self-antigen leads to a rapid decline in signaling and response sensitivity to foreign stimuli.14 Furthermore, BM-derived antigen-presenting cells (APCs) play an important role in the induction of antigen-specific immune responses of T cells.15,16 Since human T cells from mice that received only Thy/Liv (without intravenous CD34+ cells) were functional when tested by in vitro MLR assay, the lack of a suitable microenvironment in the secondary lymphoid organs that is needed for antigen presentation and T-cell priming is most likely responsible for the weak in vivo immune responses in these mice.
The novelty of this model is the combined transplantation of human thymic tissue and CD34+ cells. The human thymic graft is likely critical for preventing the development of autoimmune diseases in humanized mice. In addition to its well-known role in the induction of central tolerance, thymus also produces regulatory T (Treg) cells that are critical for suppressing self-reactive T-cell activity in the periphery.17 Recently, Hassall corpuscles were found to be essential for the development of CD4+CD25+forkhead box P3 (FOXP3)+ Treg cells in human thymus.18 The mechanism is that thymic stromal lymphopoietin (TSLP) expressed by thymic epithelial cells within the Hassall corpuscles activates thymic CD11+ DCs, and the latter mediates the secondary positive selection of CD4+CD25+FOXP3+ Treg cells. Because hematopoietic cells do not express TSLP19 and human cells do not react to mouse TSLP,20 murine thymus is unlikely to support this TSLP-DC–mediated human Treg-cell development in mice after human UCB or other hematopoietic-cell transplantation. The species specificity of TSLP may, at least in part, explain the development of autoimmunity in the thymectomized recipients of xenogeneic thymus grafts.21,22 These studies suggest that the humanized mice, in which human T cells develop in the recipient mouse thymus, are likely to have a high risk of developing autoimmune disease.
An animal model that allows de novo development and maintenance of a functional human immune system is needed not only to study normal immune functions in vivo, but also to investigate the pathophysiology of immune disorder–associated diseases. Such a model is needed for assessment of the pathogenesis of microbes, including HIV, for detailed analysis of the immune responses to infection, and for testing the efficacy of vaccines against such pathogens in vivo. Furthermore, a humanized mouse model also provides a useful means for investigating the mechanisms of transplant rejection in humans, such as xenograft rejection, for which the mechanism may differ according to donor-host species combinations. To date, xenograft rejection by human immune cells was observed only in immunodeficient mice that received in vitro preactivated human T cells.23-26 Because of the lack of in vivo T-cell priming, an essential step of T-cell activation, these models do not permit the assessment of the entire process of in vivo human cellular responses to xenoantigens. Furthermore, in such models, injected human T cells also mediate graft-versus-host responses, which may significantly alter the nature of graft rejection. In this study, we show that NOD/SCID mice that received human Thy/Liv/CD34+ FLCs were capable of rejecting skin grafts from pigs, the most suitable species for clinical xenotransplantation.27 We recently observed that these mice can also reject porcine islet xenografts (N.T., A.S., S.W., K. Yamada, G. Weir, and Y.-G. Y., manuscript in preparation). To our knowledge, this is the first humanized mouse model to show the capacity to reject porcine xenografts without the administration of preactivated human T cells.
The humanized mouse model described in this study may also be a better tool for the study of human HSCs. Because of the lack of definitive in vitro methods for identifying human HSCs, SCID-repopulating capacity has been considered a key characteristic of human HSCs. A recent study demonstrated that activated CD4+ T cells in the bone marrow play a critical role in the maintenance of normal hematopoiesis.28 The interaction between HSCs/progenitors and CD8+ cells has also been found to optimize the migration, homing, and engraftment of human HSCs/progenitors in immunodeficient mice.29 Thus, a mouse model that allows for human thymopoiesis and T-cell development may provide a better in vivo system for assessing the full potential of human HSCs than the conventional mouse model involving only human HSC transplantation.
In summary, our findings demonstrate that the addition of human CD34+ cell transplantation is essential to the establishment of a functional human immune system in immunodeficient mice that received a graft of human fetal Thy/Liv. We acknowledge that immune responses in humanized NOD/SCID mouse model may not fully represent those in humans. However, the humanized mouse model has the advantage of permitting more detailed and in-depth experiments than are possible in human subjects. In view of the sustained engraftment of human HSCs, systemic repopulation by multilineages of human lymphohematopoietic cells, and reconstitution of a functional human immune system in the mouse recipients, this model provides a useful in vivo system to study the function of human HSCs and immune cells under physiologic and pathologic conditions.
Prepublished online as Blood First Edition Paper, January 12, 2006; DOI 10.1182/blood-2005-11-4388.
Supported by a research grant from the Juvenile Diabetes Research Foundation (JDRF) (1-2005-72).
P.L. and N.T. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs William J. Burlingham, Andrew M. Tager, and Megan Sykes for critical review of the paper; Mr Orlando Moreno for outstanding animal husbandry; and Ms Luisa Raleza for expert assistance with the paper.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal