Abstract
Human intravenous immunoglobulin (IVIg) preparations are increasingly used for the treatment of autoimmune diseases. Earlier work demonstrated the presence of autoantibodies against Fas in IVIg, suggesting that IVIg might be able to induce caspase-dependent cell death in Fas-sensitive cells. In this study, we demonstrate that sialic acid–binding Ig-like lectin 9 (Siglec) represents a surface molecule on neutrophils that is activated by IVIg, resulting in caspase-dependent and caspase-independent forms of cell death. Neutrophil death was mediated by naturally occurring anti–Siglec-9 autoantibodies present in IVIg. Moreover, the efficacy of IVIg-mediated neutrophil killing was enhanced by the proinflammatory cytokines granulocyte/macrophage colony-stimulating factor (GM-CSF) and interferon-γ (IFN–γ), and this additional cell death required reactive oxygen species (ROSs) but not caspases. Anti– Siglec-9 autoantibody–depleted IVIg failed to induce this caspase-independent neutrophil death. These findings contribute to our understanding of how IVIg preparations exert their immunoregulatory effects under pathologic conditions and may provide a possible explanation for the neutropenia that is sometimes seen in association with IVIg therapy.
Introduction
Intravenous immunoglobulin (IVIg) was originally used for replacement treatment of immunodeficiency disorders associated with hypogammaglobulinemia or agammaglobulinemia. To date, IVIg preparations have been given as high-dose therapy for the treatment of autoimmune and systemic inflammatory diseases.1-3 In Guillain-Barré syndrome, idiopathic thrombocytopenic purpura, and Kawasaki disease, IVIg preparations are used as standard therapy.2,4 Despite a broad range of potential activities, the exact mechanisms of the immunoregulatory effects of IVIg are still unclear, even for approved indications.1,4-7 Therefore, a better understanding of the mechanisms of action of IVIg is essential for a more rational use of these drugs.
IVIg contains autoantibodies that react with a number of membrane molecules on immunocompetent cells relevant for the control of autoreactivity and the induction of tolerance to self.4,7 For instance, the beneficial effect of IVIg in the treatment of toxic epidermal necrolysis has been associated with the presence of autoantibodies against the Fas receptor.8-10 We have recently shown that IVIg contains blocking and agonistic antibodies and that their effect on cells is dose dependent.11 Agonistic antibodies against Fas present in IVIg have been shown to induce apoptosis in neutrophils, lymphocytes, and monocytes in a caspase-dependent manner.11-13 The cytotoxic effect on inflammatory cells may provide a potent immunoregulatory mechanism of IVIg.4
Here we show that the cytotoxic effect of IVIg on neutrophils is remarkably enhanced in the presence of certain proinflammatory cytokines and is likely in patients with inflammatory disease who undergo IVIg treatment. Subsequent analysis revealed that agonistic autoantibodies directed against the receptor, sialic acid–binding Ig-like lectin 9 (Siglec-9), are responsible for this cytokine-dependent cytotoxic effect of IVIg.
Materials and methods
Cell isolation
Neutrophils were isolated from the peripheral blood of healthy donors by Ficoll-Hypaque centrifugation.14-17 Briefly, peripheral blood mononuclear cells (PBMCs) were separated by centrifugation on Ficoll-Hypaque (Seromed-Fakola, Basel, Switzerland). The lower phase, mainly granulocytes and erythrocytes, was treated with erythrocyte lysis solution (155 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA, pH 7.3). Resultant cell populations contained greater than 95% neutrophils. Written consent was obtained from all donors, and the study was approved by the Medical Ethics Committee of the Canton Bern.
IVIg preparations
The IVIg preparation used in this study was Sandoglobulin (ZLB Behring, Bern, Switzerland). To remove the stabilizing sucrose component, the IVIg preparation was dialyzed with a Spectra/Por 3 membrane (standard cellulose dialyze tubing; molecular weight cutoff, 3500 Da) obtained from Spectrum Medical Industries (Los Angeles, CA). F(ab′)2 fragments of IVIg preparations were produced by digestion and subsequent purification using a protein A–agarose column and an ImmunoPure F(ab′)2 preparation kit (Pierce Chemical, Rockford, IL). The purity of the F(ab′)2 fragments was confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
Depletion of anti–Siglec-9 and potential anti–Siglec-3 (CD33) antibodies (Abs) from IVIg preparations was performed by affinity chromatography.18 Briefly, recombinant Siglec-9/Fc and SIGLEC3/Fc fusion proteins (R&D Systems, distributed by Bühlmann, Basel, Switzerland) were coupled to Affi-Gel beads (Bio-Rad Laboratories, Reinach, Switzerland). The coupled beads were incubated with IVIg on a rotator at 4°C overnight. After a centrifugation step, the IVIg fractions depleted of potential anti-Siglec Abs were collected. To recover IVIg-derived anti–Siglec-9 Abs, coated beads were incubated in the presence of 0.1 M glycine-HCl, pH 2.7 (Riedel-DeHäen, Seelze, Germany) on a rotating wheel for 15 minutes. After centrifugation, the supernatant containing the anti–Siglec-9-specific Abs was separated from the beads and neutralized with 1 M NaOH. Protein concentration and buffer exchange to PBS were performed in 10 000-Da cutoff concentrator tubes (Vivaspin; Vivascience, Göttingen, Germany). Protein concentrations were determined with a spectrophotometer (ND-1000; NanoDrop Technologies, Wilmington, DE). The content of aggregate formation was tested by high-performance liquid chromatography (HPLC) and was found to be less than 2% for all IVIg fractions. For HPLC, a TSK 3000 column (Tosho, Tokyo, Japan) and an HP Ti Series 1050 Autosampler (Hewlett-Packard, Palo Alto, CA) were used.
Immunofluorescence
Binding of human anti–Siglec-9 Abs purified from IVIg to neutrophils was analyzed by flow cytometry (FACSCalibur; Becton Dickinson Biosciences, Basel, Switzerland). Fcγ receptors were blocked with a mixture of antihuman mAb F(ab′)2 fragments from anti-CD16 (clone 3G8), anti-CD32 (clone 7.3), and anti-CD64 (clone 10.1) (all from Ancell, distributed by Alexis, Lausen, Switzerland), as previously described.19 Neutrophils were then incubated with saturating concentrations of human anti–Siglec-9 Abs purified from IVIg or anti–Siglec-9 mAb (clone E10-286; Becton Dickinson Biosciences). Binding of the primary Abs was visualized with the following secondary Abs: phycoerythrin (PE)–conjugated F(ab′)2 fragments of goat anti–human IgG + IgM (H + L) and goat anti–mouse IgG (Jackson ImmunoResearch Laboratories, distributed by Milan Analytica, La Roche, Switzerland).
Cell cultures
Cells were cultured at 1 × 106/mL in the presence or absence of cytokines, Abs, or both, for the indicated times using complete culture medium (RPMI 1640 containing 10% FCS and 200 IU/mL penicillin/100 μg/mL streptomycin; Life Technologies, Basel, Switzerland). Unless otherwise indicated, cells were stimulated with 20 mg/mL IVIg (133.3 μM). F(ab′)2 fragments of IVIg were used at 133.3 μM. Fcγ receptors were blocked with a mixture of antihuman mAb F(ab′)2 fragments from anti-CD16 (clone 3G8), anti-CD32 (clone 7.3), and anti-CD64 (clone 10.1) (see “Immunofluorescence”). These mAb F(ab′)2 fragments were used at 600 nM and added 30 minutes before the addition of IVIg. Cytokine stimulation occurred 25 minutes before the addition of IVIg. GM-CSF (25 ng/mL; Novartis Pharma GmbH, Nürnberg, Germany), G-CSF (25 ng/mL; Aventis Pharma, Zurich, Switzerland), IFN-γ (85 ng/mL R&D Systems), caspase inhibitor N-benzyloxycarbonyl (z)–Val-Ala-Asp (VAD)–fluoromethylketone (fmk, 50 μM; Becton Dickinson Biosciences), and N-acetyl-l-cysteine (NAC, 10 μM; Sigma-Aldrich Chemie GmbH, Buchs SG, Switzerland) were used. For nicotinamide adenine dinucleotide phosphate (NADPH) oxidase–inhibition experiments, neutrophils were incubated with 20 μM diphenyleneiodonium (DPI; Calbiochem, distributed by Juro Supply GmbH, Lucerne, Switzerland) at 37°C for 5 minutes and then washed twice with RPMI 1640 before culture.
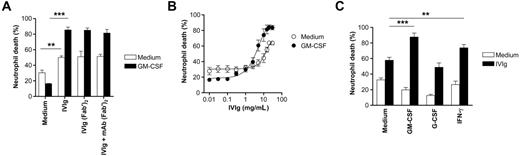
IVIg accelerates spontaneous neutrophil death, which is enhanced after priming with GM-CSF or IFN-γ. Cell death was assessed by ethidium bromide uptake and flow cytometric analysis. (A) Acceleration of neutrophil death by treatment with IVIg or F(ab′)2 fragments of IVIg. IVIg effects were also determined in the presence of mAb F(ab′)2 fragments to block Fcγ receptors. Results of 24-hour cultures are shown (n = 4). **P < .01. ***P < .001. No statistical difference was seen between the different IVIg stimulation conditions. (B) Concentration-effect curve of IVIg in 20-hour neutrophil cultures. Maximal death effects in the absence of cytokine priming were seen at 20 mg/mL. GM-CSF, at low concentrations of IVIg, protected cells from spontaneous apoptosis, whereas it promoted death at higher concentrations (n = 3). (C) GM-CSF and IFN-γ, but not G-CSF, were able to enhance IVIg-mediated neutrophil death. Results of 24-hour cultures are shown (n = 4). **P < .01; ***P < .001.
IVIg accelerates spontaneous neutrophil death, which is enhanced after priming with GM-CSF or IFN-γ. Cell death was assessed by ethidium bromide uptake and flow cytometric analysis. (A) Acceleration of neutrophil death by treatment with IVIg or F(ab′)2 fragments of IVIg. IVIg effects were also determined in the presence of mAb F(ab′)2 fragments to block Fcγ receptors. Results of 24-hour cultures are shown (n = 4). **P < .01. ***P < .001. No statistical difference was seen between the different IVIg stimulation conditions. (B) Concentration-effect curve of IVIg in 20-hour neutrophil cultures. Maximal death effects in the absence of cytokine priming were seen at 20 mg/mL. GM-CSF, at low concentrations of IVIg, protected cells from spontaneous apoptosis, whereas it promoted death at higher concentrations (n = 3). (C) GM-CSF and IFN-γ, but not G-CSF, were able to enhance IVIg-mediated neutrophil death. Results of 24-hour cultures are shown (n = 4). **P < .01; ***P < .001.
Determination of cell death and apoptosis
Measurement of Siglec-9–Ig binding affinity using surface plasmon resonance
The Siglec-9/Fc fusion protein was covalently coupled to a CM5 sensor chip through amine ester linkage through the activation of chip surface dextran carboxyl groups by incubation with a mixture of 1-ethyl-3-(3-dimethylaminopropyl)–carbodiimide and N-hydroxysuccinimide (all Biacore AB, Uppsala, Sweden). Repeated manual injections of 2 μL Siglec-9 fusion protein in 10 mM sodium acetate (final concentration, 20 μg/mL) were performed until the immobilization level corresponding to approximately 2500 response units (RUs) was reached. Subsequently, 1 M ethanolamine-HCl, pH 8.5, was injected to deactivate the remaining reactive carboxyl groups, and excess ligand was removed by regeneration with 10 mM NaOH. For analysis, all Abs were diluted to the indicated concentrations in HBS-EP buffer (Biacore) containing 0.01 M HEPES, pH 7.4, 0.15 M NaCl, 3 mM EDTA, and 0.005% surfactant P20 and were measured on a Biacore X instrument (Biacore). After binding to the biosensor chip surface, the assayed Abs were removed by regeneration with 10 mM NaOH until baseline response values were reached. Data were recorded and analyzed with BIAsimulation software (Biacore).
Statistical analysis
Statistical analysis was performed with the Student t test. If mean levels are presented, the standard error of the mean (SEM), and the number (n) of independent experiments is indicated in each case. P values below .05 were considered statistically significant.
Results
IVIg preparations induce neutrophil death, which is accelerated by the proinflammatory cytokines GM-CSF and IFN-γ
Previously, we have shown that concentrations of IVIg higher than 20 mg/mL are cytotoxic for neutrophils.11 In this study, we assessed IVIg-mediated neutrophil death in the presence and absence of inflammatory cytokines in vitro. We found that simultaneous exposure of neutrophils to GM-CSF remarkably increased IVIg-mediated cytotoxicity. This increased cytotoxicity was most efficient if cells were preincubated with GM-CSF for 25 minutes before a death-inducing concentration of IVIg (20 mg/mL) was added (Figure 1A). The IVIg effects were unlikely mediated by Fcγ receptors present on neutrophils because F(ab′)2 fragments of IVIg demonstrated the same cytotoxic effects. Moreover, blocking of the Fcγ receptors using a mixture of mAb F(ab′)2 fragments19 did not prevent IVIg-mediated neutrophil death in the presence or absence of GM-CSF. Concentration-dependent experiments showed that GM-CSF increased the efficacy and the potency of IVIg-mediated neutrophil cytotoxicity (Figure 1B). The concentration-dependent IVIg-mediated switch from cellular protection to promotion of cell death by GM-CSF was a surprising finding because this cytokine is generally considered a survival factor for neutrophils.21-24
We tested additional cytokines for their capacity to enhance the cytotoxic activity of IVIg on neutrophils. As with GM-CSF, short-term exposure to IFN-γ also increased IVIg-mediated neutrophil death. In contrast, the neutrophil survival factor G-CSF had no effect on the cytotoxicity of IVIg (Figure 1C). Thus, it appears that the increased efficacy and potency of IVIg-related cytotoxicity was limited to a subset of cytokines and not linked to their capacity to increase neutrophil survival.
IVIg activates caspase- and reactive oxygen species–dependent death pathways in neutrophils
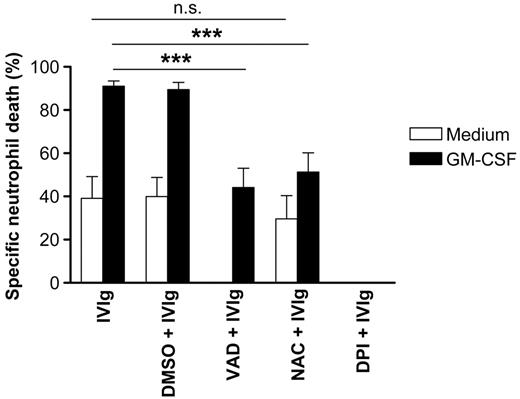
We next investigated whether IVIg-mediated neutrophil death in the presence or absence of cytokines resulted from caspase activation. Caspases play a critical role in the execution phase of neutrophil apoptosis.25 In the absence of GM-CSF, the pan-caspase inhibitor z-VAD-fmk completely blocked IVIg-mediated cell death, suggesting that caspases play a role in death execution. However, in GM-CSF–preincubated neutrophils, IVIg-mediated cell death was only partially blocked by the caspase inhibitor, pointing to the possibility that caspase-independent death pathways are activated under these conditions in at least a subpopulation of dying neutrophils (Figure 2).
ROSs have previously been described as regulators of apoptosis and necrosis in several cellular systems.26 NAC, a well-characterized ROS scavenger, had little effect on IVIg-mediated death in the absence of GM-CSF (Figure 2). However, strong inhibition of IVIg-mediated neutrophil death by NAC was seen in the presence of GM-CSF. The antideath activity of NAC was dose dependent and reached its maximum at 10 μM (data not shown). In neutrophils, ROSs are generated primarily by the inducible nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system. To further investigate the potential role of ROSs in IVIg-mediated neutrophil death, we used the NADPH oxidase-inhibitor DPI. In contrast to the findings of the ROS scavenger studies with NAC, blocking of ROS synthesis by DPI was more efficient and completely abrogated IVIg-mediated death in the presence and the absence of GM-CSF (Figure 2).
Evidence for caspase-dependent and ROS-dependent pathways of IVIg-mediated death. The caspase inhibitor z-VAD-fmk blocked IVIg-mediated neutrophil death completely. However, the enhanced IVIg-triggered death after GM-CSF preincubation was only partially blocked by the caspase inhibitor. The ROS scavenger NAC blocked IVIg-mediated death in GM-CSF–primed neutrophils, whereas the inhibitor of ROS production, DPI, completely blocked IVIg-mediated death in the absence and presence of GM-CSF. Results of 20-hour cultures are shown (n = 3). ***P < .001.
Evidence for caspase-dependent and ROS-dependent pathways of IVIg-mediated death. The caspase inhibitor z-VAD-fmk blocked IVIg-mediated neutrophil death completely. However, the enhanced IVIg-triggered death after GM-CSF preincubation was only partially blocked by the caspase inhibitor. The ROS scavenger NAC blocked IVIg-mediated death in GM-CSF–primed neutrophils, whereas the inhibitor of ROS production, DPI, completely blocked IVIg-mediated death in the absence and presence of GM-CSF. Results of 20-hour cultures are shown (n = 3). ***P < .001.
Given that we previously observed caspase-independent but ROS-dependent autophagic-like cell death in anti–Siglec-9 mAb–treated neutrophils,14 we investigated the morphology of neutrophils on concurrent exposure to GM-CSF and IVIg and observed multiple cells exhibiting cytoplasmic vacuolization (15-hour cultures; data not shown), confirming that the type of neutrophil death under these conditions was largely nonapoptotic. Based on these data, we hypothesized that IVIg may contain anti–Siglec-9 autoantibodies.
IVIg preparations contain autoantibodies against Siglec-9
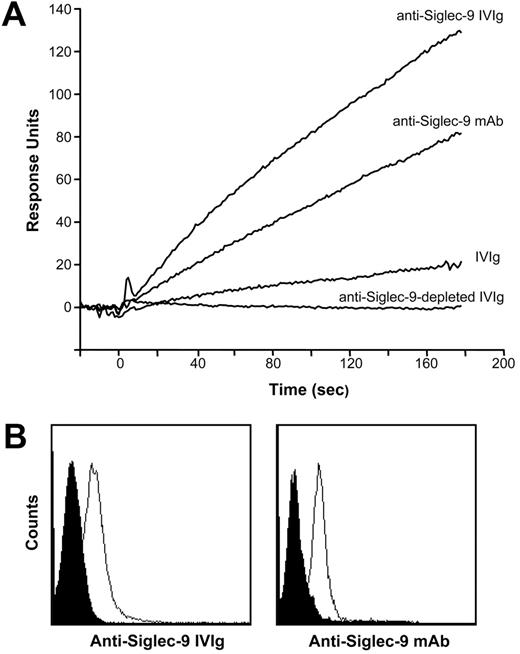
To identify these antibodies, we applied surface plasmon resonance to investigate the binding capacity of IVIg to a recombinant Siglec-9/Fc fusion protein covalently immobilized on a solid phase. Initial nephelometric experiments confirmed the binding specificity of IVIg to Siglec-9 in a concentration-dependent manner (500 nM-7.5 μM), whereas there was no detectable reactivity to the human IgG1 Fc fusion partner of the Siglec-9/Fc protein (data not shown). We then performed kinetic and affinity experiments with IVIg fractions that were depleted (anti–Siglec-9–depleted IVIg) or enriched with anti–Siglec-9 autoantibodies (anti–Siglec-9 IVIg) (see “IVIg preparations”). To exclude nonspecific binding effects, parallel signals produced by a noncoated but otherwise identical flow cell were continuously subtracted from the primary curves. An anti–Siglec-9 mAb was used as a positive control and showed stable binding to the Siglec-9/Fc protein.
Figure 3A depicts the association curves of the tested fractions at 1 μM. Affinity-purified anti–Siglec-9 Abs demonstrated a marked increase in the association curve compared with that of total IVIg. In contrast, anti–Siglec-9–depleted IVIg showed no evidence of binding to the Siglec-9/Fc fusion protein, suggesting that Abs recognizing Siglec-9 were successfully removed in this IVIg fraction. For the natural anti–Siglec-9 autoantibodies present in IVIg, we calculated a dissociation constant Kd of 1.27 × 10–6 M. Moreover, the human anti–Siglec-9 Abs purified from IVIg bound to blood neutrophils, as assessed by flow cytometry (Figure 3B), suggesting that these autoantibodies also bind to surface Siglec-9 on cells.
Autoantibodies against Siglec-9 are responsible for the increased cytotoxicity of IVIg in the presence of GM-CSF
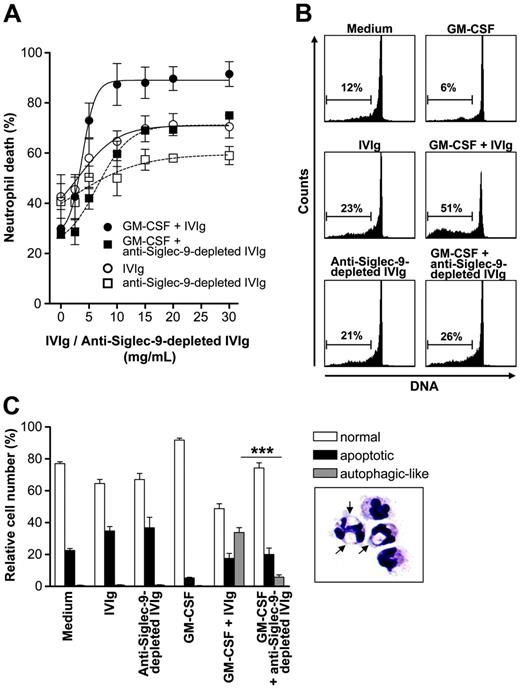
We next investigated the death-inducing capacity of the IVIg fraction depleted of anti–Siglec-9 autoantibodies. In the absence of GM-CSF, anti–Siglec-9–depleted IVIg had little effect on neutrophil viability. Moreover, the strong death-inducing capacity of IVIg in the presence of GM-CSF was essentially absent if anti–Siglec-9–depleted IVIg was used (Figure 4A). Besides diminished death efficacy, anti–Siglec-9–depleted IVIg had less death potency because, compared with total IVIg, much higher concentrations were needed to detect a cytotoxic effect on neutrophils. No difference compared with normal IVIg was seen in a fraction, which was depleted from potential anti-CD33 (Siglec-3) autoantibodies and used as an additional control (data not shown).
To further demonstrate the dependence on anti–Siglec-9 autoantibodies in IVIg preparations for the nonapoptotic neutrophil death in the presence of GM-CSF, we analyzed DNA fragmentation using a flow cytometric assay. DNA fragmentation, which is associated with spontaneous apoptosis,15-17 was enhanced on treatment with IVIg and anti–Siglec-9–depleted IVIg. The cell death induced under these conditions may be partially attributed to the presence of agonistic anti-Fas autoantibodies.11-13 In the presence of GM-CSF, the generation of hypoploid DNA by IVIg was further elevated (Figure 4B). In contrast, almost no increased DNA fragmentation was observed in GM-CSF–pretreated neutrophils if the anti–Siglec-9–depleted IVIg fraction was used.
IVIg contained antibodies against human Siglec-9 and bound to neutrophils, as assessed by surface plasma resonance (SPR) and flow cytometry. (A) Sensorgrams of the binding activity of different fractions of IVIg (all at 1 μM) and a control anti–Siglec-9 mAb (1 nM) to immobilized recombinant Siglec-9/Fc fusion protein are shown. Binding of total IVIg to Siglec-9 was detected, confirming the existence of autoantibodies against Siglec-9 in IVIg (anti–Siglec-9 IVIg). In contrast, anti–Siglec-9–depleted IVIg did not show any binding to Siglec-9, suggesting that autoantibodies against Siglec-9 were successfully depleted from this fraction. In comparison, affinity-purified anti–Siglec-9 IVIg showed a marked increase in the association curve. (B) Freshly isolated mature blood neutrophils were analyzed by flow cytometry. Purified human anti–Siglec-9 Abs and mouse anti–Siglec-9 mAb (clone E10-286) similarly bound to the cell surfaces of neutrophils (black peak: control staining; white peak: anti–Siglec-9 staining). Representative examples of 4 independent experiments are shown.
IVIg contained antibodies against human Siglec-9 and bound to neutrophils, as assessed by surface plasma resonance (SPR) and flow cytometry. (A) Sensorgrams of the binding activity of different fractions of IVIg (all at 1 μM) and a control anti–Siglec-9 mAb (1 nM) to immobilized recombinant Siglec-9/Fc fusion protein are shown. Binding of total IVIg to Siglec-9 was detected, confirming the existence of autoantibodies against Siglec-9 in IVIg (anti–Siglec-9 IVIg). In contrast, anti–Siglec-9–depleted IVIg did not show any binding to Siglec-9, suggesting that autoantibodies against Siglec-9 were successfully depleted from this fraction. In comparison, affinity-purified anti–Siglec-9 IVIg showed a marked increase in the association curve. (B) Freshly isolated mature blood neutrophils were analyzed by flow cytometry. Purified human anti–Siglec-9 Abs and mouse anti–Siglec-9 mAb (clone E10-286) similarly bound to the cell surfaces of neutrophils (black peak: control staining; white peak: anti–Siglec-9 staining). Representative examples of 4 independent experiments are shown.
Caspase-independent neutrophil death has previously been shown to be associated with an autophagic morphology because the cells demonstrated large cytoplasmic vacuoles.14 We therefore carefully analyzed the morphology of neutrophils in the presence and absence of GM-CSF and the different IVIg fractions. Aberrant cell death associated with large cytoplasmic vacuoles was seen in GM-CSF–pretreated and IVIg-stimulated neutrophils. Quantitative analysis revealed that this form of cell death was significantly reduced when anti–Siglec-9 autoantibodies were removed from IVIg (Figure 4C).
Discussion
In this study, we provide evidence for the existence of autoantibodies directed against Siglec-9 in IVIg, suggesting that healthy persons have autoreactivity against this receptor. Moreover, it should be noted that our observations could be considered the first known physiological receptor interaction resulting in a functional signal for a member of the CD33-like subgroup of SIGLECs. However, Siglec-9 is not the first molecule that serves as an autoantigen under physiological conditions. For instance, we and others11-13 reported that IVIg contains anti-Fas autoantibodies. Other known examples of autoantigens are CD4,27 MHC class I,28 and cytokines.29 It has been suggested that such natural autoantibodies may have tissue homeostatic functions.30
The first evidence for the existence of natural anti–Siglec-9 autoantibodies was obtained from functional studies using neutrophils as target cells. We observed that IVIg exhibited high caspase-independent neutrophil cytotoxicity in the presence of some, but not all, proinflammatory cytokines and reported this phenomenon after Siglec-9 stimulation of neutrophils in vitro.14 Thus, we hypothesized that IVIg contains agonistic anti–Siglec-9 autoantibodies and generated anti– Siglec-9–depleted IVIg to perform binding and functional studies in comparison with total IVIg. In the functional studies, it was not possible to use the purified anti–Siglec-9 autoantibody fraction that we used in the binding assays because the yield after purification was insufficient and did not allow functional studies. Nevertheless, the available data obtained by using anti–Siglec-9–depleted IVIg appeared to be sufficient to suggest that the additional and caspase-independent neutrophil death in the presence of GM-CSF was largely mediated by anti–Siglec-9 autoantibodies present in IVIg. Whether these Abs may also exhibit a pathologic role in autoimmunity remains to be determined. In such future studies, it would be interesting to somehow quantify anti–Siglec-9 autoantibodies in single sera of patients with inflammatory disease.
The results of the current study have several implications for IVIg treatment. For instance, the increased efficacy and potency of IVIg in a cytokine-rich environment might explain the local anti-inflammatory effects of systemically applied IVIg. After diffusion from the intravascular compartment to the site of inflammation, the concentrations of IVIg are likely to be reduced but may still be sufficient to induce neutrophil cell death, which may contribute to the resolution of inflammation. On the other hand, the reason for IVIg-induced neutropenia in some patients remains obscure,31-35 and the results of our study may also provide a potential mechanism of this unwanted effect of IVIg therapy. In particular, under inflammatory conditions, IVIg-induced neutropenia may be mediated by natural anti–Siglec-9 autoantibodies. Future work is required to distinguish those patients who may benefit from IVIg from those who may carry a risk for unwanted effects associated with such treatment. Moreover, the natural ligand of Siglec-9 remains to be identified.
Anti–Siglec-9 autoantibodies are responsible for the increased cytotoxicity of IVIg in the presence of GM-CSF. (A) Concentration-effect curves of total IVIg and anti–Siglec-9–depleted IVIg, respectively, in 20-hour neutrophil cultures. In the absence of cytokine pretreatment, the efficacy of anti–Siglec-9–depleted IVIg was similar to total IVIg, but its potency was reduced. Depletion of anti–Siglec-9 Abs from the IVIg preparation resulted in loss of the GM-CSF–mediated increase of death efficacy and potency (n = 3). In these experiments, we also used IVIg depleted from potential anti–Siglec-3 Abs that exhibited no difference compared with complete IVIg (data not shown). (B) DNA fragmentation assay. Both IVIg and anti–Siglec-9–depleted IVIg induced DNA fragmentation in the absence of GM-CSF. In the presence of GM-CSF, IVIg treatment resulted in enhanced DNA fragmentation that was not seen if anti–Siglec-9–depleted IVIg was used. Results of 13-hour cultures are shown. (C) Light microscopy. Both IVIg and anti–Siglec-9–depleted IVIg accelerated neutrophil apoptosis (reduced cell volume and nuclear condensation) in a similar manner. In the presence of GM-CSF, IVIg treatment resulted in a subpopulation of cells demonstrating cytoplasmic vacuolization (typical cells are shown in the lower right panel; arrows indicate vacuoles; original magnification, 1000×) that underwent autophagic-like cell death. This type of cell death was significantly reduced when GM-CSF–pretreated neutrophils were stimulated with anti–Siglec-9–depleted IVIg. Results of 15-hour cultures are shown (n = 3). ***P < .001. Image was captured with an Axiovert 63×/1.4 NA oil objective lens (Carl Zeiss, Heidelberg, Germany) and was processed with Adobe Photoshop 5.0 software (Adobe, San Jose, CA).
Anti–Siglec-9 autoantibodies are responsible for the increased cytotoxicity of IVIg in the presence of GM-CSF. (A) Concentration-effect curves of total IVIg and anti–Siglec-9–depleted IVIg, respectively, in 20-hour neutrophil cultures. In the absence of cytokine pretreatment, the efficacy of anti–Siglec-9–depleted IVIg was similar to total IVIg, but its potency was reduced. Depletion of anti–Siglec-9 Abs from the IVIg preparation resulted in loss of the GM-CSF–mediated increase of death efficacy and potency (n = 3). In these experiments, we also used IVIg depleted from potential anti–Siglec-3 Abs that exhibited no difference compared with complete IVIg (data not shown). (B) DNA fragmentation assay. Both IVIg and anti–Siglec-9–depleted IVIg induced DNA fragmentation in the absence of GM-CSF. In the presence of GM-CSF, IVIg treatment resulted in enhanced DNA fragmentation that was not seen if anti–Siglec-9–depleted IVIg was used. Results of 13-hour cultures are shown. (C) Light microscopy. Both IVIg and anti–Siglec-9–depleted IVIg accelerated neutrophil apoptosis (reduced cell volume and nuclear condensation) in a similar manner. In the presence of GM-CSF, IVIg treatment resulted in a subpopulation of cells demonstrating cytoplasmic vacuolization (typical cells are shown in the lower right panel; arrows indicate vacuoles; original magnification, 1000×) that underwent autophagic-like cell death. This type of cell death was significantly reduced when GM-CSF–pretreated neutrophils were stimulated with anti–Siglec-9–depleted IVIg. Results of 15-hour cultures are shown (n = 3). ***P < .001. Image was captured with an Axiovert 63×/1.4 NA oil objective lens (Carl Zeiss, Heidelberg, Germany) and was processed with Adobe Photoshop 5.0 software (Adobe, San Jose, CA).
Authorship
Contribution: S.v.G. designed research, performed research, collected data, analyzed data, and wrote the paper. A.S. performed research, collected data, and analyzed data. M.V. performed research. B.M.S. contributed analytical tools. S.M. contributed analytical tools and wrote the paper. H.-U.S. designed research, contributed reagents and analytical tools, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 10, 2006; DOI 10.1182/blood-2006-05-021568.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Drs D. Simon (Department of Dermatology, University of Bern) and M. Neef (Department of Clinical Pharmacology, University of Bern) for the organization of blood samples.
This work was supported by the Swiss National Science Foundation (grant 310000-107526) and the OPO Foundation, Zurich.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal