Abstract
Paracellular diapedesis, a key step in leukocyte recruitment to the site of inflammation, occurs at endothelial junctions and is regulated by highly coordinated interactions between leukocytes and endothelium. We found that CD157, a glycosylphosphatidylinositol-anchored ectoenzyme belonging to the NADase/ADP-ribosyl cyclase family, plays a crucial role for neutrophil diapedesis, because its ligation with specific monoclonal antibodies (both on neutrophils or endothelial cells) results in altered neutrophil movement on the apical surface of endothelium and, ultimately, in loss of diapedesis. Real-time microscopy revealed that CD157 behaves as a sort of compass during the interaction between neutrophils and endothelial cells; indeed, following CD157 ligation, neutrophils appear disoriented, meandering toward junctions where they eventually stop without transmigrating. These findings are relevant in vivo because CD157-deficient neutrophils obtained from patients with paroxysmal nocturnal hemoglobinuria are characterized by a severely impaired diapedesis.
Introduction
Leukocyte extravasation, a crucial step in immune responses and inflammatory reactions, is governed by sequential molecular interactions between leukocytes circulating in the bloodstream and endothelial cells lining the vascular wall.1,2 This cascade involves initial tethering and rolling steps, prevalently mediated by selectin-based adhesive events3 and a subsequent firm adhesion mediated by integrins.4 Then, leukocytes polarize, move to endothelial cell junctions, and migrate through them in sequential processes referred to as locomotion and transendothelial migration (or diapedesis), respectively.5,6
In contrast to leukocyte rolling and firm adhesion to endothelium, the molecular details of leukocyte diapedesis have not been completely defined. A small number of molecules have been identified that control the different steps of neutrophil and monocyte transendothelial migration; these include CD31, CD99, VE-cadherin, and junctional adhesion molecules (JAM-1, -2, and -3).7-10 However, data derived from in vitro and in vivo models suggest that other molecules are involved in the process of diapedesis.11,12 On molecular identification of these, several, unexpectedly, turned out to be ectoenzymes.13 We addressed our attention to CD157 surface enzyme, a glycosylphosphatidylinositol (GPI)–linked molecule encoded by a member of the NADase/ADP-ribosyl cyclase (cADPR) gene family, which also includes CD38.14-16 The findings that CD157 is expressed both by neutrophils and endothelial cells and is implicated in neutrophil chemotaxis17 were highly indicative of potential involvement in transendothelial migration and, eventually, in neutrophil recruitment to the inflammatory site.
This work demonstrates that CD157 is (1) constitutively expressed at vascular interendothelial junctions and its expression is not affected by cell activation, (2) is crucial to transendothelial migration of neutrophils, and (3) orchestrates neutrophil journey through endothelial barrier. These findings are consistent with the results obtained in neutrophils from patients with paroxysmal nocturnal hemoglobinuria (PNH), whose CD157 is deficient; indeed, these neutrophils show constant defects in transendothelial migration.
Materials and methods
Antibodies and reagents
Anti-HLA class I monoclonal antibody (mAb) O1.65 (IgG2a), anti-CD31 mAb Moon-1 (IgG1),18 and anti-CD18 mAb TS1/18 (IgG1, provided by A. Arnout, Boston University, Boston, MA) were purified and depleted of endotoxin, as described.19 Anti-CD157 mAbs RF3, Bec-7, and SG2 (all IgG1) were provided by K. Ishihara and T. Hirano (University of Osaka, Osaka, Japan)20 ; mAb Mo5 (IgG1) was provided by R. Todd 3rd (University of Michigan Health System, Ann Arbor, MI)21 ; and anti–ICAM-1 mAbs MEM-111 and MEM-112 (IgG1) were a gift from V. Horejsi (Academy of Sciences of the Czech Republic, Prague, Czech Republic). Affinity-purified, F(ab′)2 fraction of goat antibody to mouse immunoglobulin labeled with FITC (F(ab′)2-GαMIg-FITC), Texas red (F(ab′)2-GαMIg-Texas red), or Cy2 (F(ab′)2-GαMIg-Cy2) and purified murine IgG Fc fragments were from Jackson ImmunoResearch (West Grove, PA).
Formyl-methionyl-leucyl-phenylalanine (fMLP), fibronectin, PMA, LPS, and dibutyryl-cAMP were from Sigma (Milano, Italy). Carboxyfluorescein diacetate succinimidyl ester (CFSE) was from Molecular Probes (Invitrogen, Milano, Italy), and TNF-α, IL-1β, IFN-α, IFN-γ, and IL-4 were from PeproTech (London, United Kingdom).
Endothelial cells
Human umbilical vein endothelial cells (HUVECs) were isolated from human umbilical veins and grown by standard methods.22 Experiments were routinely performed on cells at passage 2. In selected experiments, TNF-α (20 ng/mL) was added for the final 4 to 8 hours.
Isolation of human polymorphonuclear cells
Peripheral blood was obtained from healthy blood donors or patients with PNH and centrifuged through Ficoll-Paque (Amersham Pharmacia, Milano, Italy). Polymorphonuclear cells (PMNs) were isolated to more than 95% purity as previously described.17
Informed consent was obtained from patients with PNH enrolled in the study before each blood sample collection, according to institutional guidelines and the Declaration of Helsinki.
Immunofluorescence
Adherent HUVECs were suspended for flow cytometry by incubation with 0.75 mM EDTA, washed and incubated for 30 minutes at 4°C with the selected mAb (5 μg/mL), then washed and incubated with F(ab′)2-GαMIg-FITC. Fluorescence was analyzed by means of a FACSCalibur flow cytometer using CellQuest software (Becton Dickinson, Milano, Italy). Background mAb binding was estimated by means of isotype-matched negative control mAb.
In confocal microscopy experiments, HUVECs were grown until confluence on coverslips coated with fibronectin (10 μg/mL). Then, they were fixed with 2% paraformaldehyde (PFA), washed, incubated in PBS with 20% FCS for 30 minutes at 20°C, and stained with mAb (10 μg/mL) for 30 minutes and with F(ab′)2-GαMIg-Cy2 for 15 minutes.
Western blot
PMNs and HUVECs were lysed and proteins separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 10%) under reducing conditions and transferred to Immunoblot PVDF membrane. After blocking, membranes were probed with anti-CD157 mAb followed by horseradish peroxidase-conjugated anti–mouse IgG. The immunoreactive bands were detected by enhanced chemiluminescence reagent (Western lightning Chemiluminescence Reagent Plus, Perkin Elmer Life Sciences, Monza, Italy).
Transendothelial migration assays
PMN transmigration was performed as described23 with minor modifications. Briefly, HUVECs were grown to confluence on transwell filters (3 μm pore size) coated with fibronectin (10 μg/mL). At the beginning of the assay, 1 mL RPMI 1640 containing fMLP (10 nM) was added to the lower compartment. PMNs (106/mL) pretreated with purified murine IgG Fc fragments (150 μg/mL) to block Fc receptor were seeded on HUVEC monolayers. In selected experiments, mAbs (20 μg/mL) were incubated with PMNs or HUVECs or both for 20 minutes before the assay, then removed or left, as indicated. After incubation (1 hour at 37°C, unless otherwise reported), the percentage of transmigrated cells was determined by counting cells in the lower compartment and comparing the number with the total number of cells added.
Confocal microscopy of transendothelial migration was assessed on HUVECs grown until confluence on collagen. Briefly, 230 μL collagen (1.2 mg/mL) in RPMI 1640 medium containing 0.1 M acetic acid, 0.02 M HEPES, and 0.04 M NaOH was added on coverslips and incubated for 3 hours at 37°C. CFSE-labeled PMNs (5 × 105) treated with the indicated mAb (20 μg/mL) were plated on HUVECs and incubated for 30 minutes at 37°C. After fixation with 2% PFA, nonadherent PMNs were carefully washed away. Samples were incubated with PBS containing 20% FCS for 30 minutes and stained with anti-CD31 mAb and F(ab′)2-GαMIg-Texas red.
Samples were analyzed using an Olympus FV300 (Olympus Biosystems, Milano, Italy) confocal microscope equipped with a green helium neon (543 nm) laser, a blue argon (488 nm) laser, and FluoView 300 software (Olympus Biosystems). Cells were imaged using a ×60 oil immersion objective (1.4 NA). Samples were analyzed by sequential scanning of the xy planes recorded along the z-axis (step size: 1 μm). Series of confocal optical xy images were processed using a 3-dimensional reconstruction program (FluoView 300 software) and visualized as orthogonal views.
Endothelial cell junctions were stained as described.24 Briefly, HUVEC-PMN cocultures were fixed with PBS containing 0.05% glutaraldehyde for 10 minutes, washed, and incubated with 0.25% AgNO3 for 40 seconds. The silver stain was developed under UV light for 5 minutes.
PMN adhesion assay
HUVECs were grown until confluence on fibronectin-coated 96-well plates. 51Cr-labeled neutrophils (pretreated with 150 μg/mL affinity-purified murine Fc fragment) were incubated with the mAb indicated (20 μg/mL) for 20 minutes at 4°C, then added to HUVECs and allowed to adhere. Nonadherent PMNs were washed off, and adherent cells were lysed with 2% Triton X-100. The counts per minute (cpm) in the lysates were determined in a γ counter. Percent specific adhesion was calculated following the formula: (51Cr released from adherent cells/total 51Cr added to each well) × 100.
Analysis of neutrophil movement using time-lapse videomicroscopy
PMN migration was visualized in real time on HUVECs grown on collagen. Nomarski differential interference contrast (DIC) images were captured using an F-View II CCD camera connected to an Olympus IX70 inverted microscope controlled by CellR imaging software (Olympus Biosystems, Milano, Italy). Samples were placed on a heated stage (Thermoplate, Tokai Hit, Olympus Biosystems) set at 37°C and images were taken using a 40×/0.60 NA LucPlanFLN objective. PMNs were added on HUVEC monolayers, and frames were captured every 10 seconds for 40 minutes. Frames were then combined in stacks and exported in AVI format as movies. All the images were processed using the TrackIt! software (Olympus Biosystems) to measure the trajectory lengths between the site of neutrophil-HUVEC interaction and the site of diapedesis.
Statistical analysis
Values are expressed as mean ± SD (unless otherwise reported). Statistical significance was assessed by the Student t test for normal distributed variables or with the Mann-Whitney U test for nonnormal variables. P values below .05 were considered statistically significant (GraphPad Prism 4.03, San Diego, CA).
Results
CD157 expression by human endothelial cells
Flow cytometric analysis demonstrated that CD157 is expressed at low epitope density by virtually all resting HUVECs (Figure 1A) and has an apparent molecular weight of about 45 kDa (Figure 1C). Confocal microscopy analysis showed that CD157 has a typical spot pattern and is prevalently localized at the intercellular junctions of confluent HUVEC monolayers. A weak diffuse expression is appreciable at the basal surface (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). A mAb specific for CD54/ICAM-1, used as a control, produced a more diffuse surface staining, whereas a mAb to CD31/PECAM-1 showed the expected reactivity restricted to the cell borders (Figures 1B and S1). These results confirm that the CD157 distribution is specific and is not an artifact of the staining procedure.
Surface expression and distribution of CD157 was not affected by activation of HUVECs by different cytokines (TNF-α, IL1-β, IFN-α, IFN-γ, and IL-4) and by chemical mediators (fMLP, LPS, PMA, and dibutyryl-cAMP) at any incubation time considered. As expected, activation markedly increased CD54 expression (Figure 1D).25
Analysis of CD157 expression. (A) Flow cytometric analysis of the surface expression of CD157, CD54, and CD31 on HUVECs. Gray-filled profiles are the isotype-control mAb. Number of events was 10 000. Results are representative of 5 experiments. (B) Confluent HUVEC monolayers were stained and the expression of CD157, CD54, and CD31 analyzed by laser-scanning confocal microscopy. Cells were imaged using a ×60/1.4 numerical aperture (NA) oil immersion objective. Bar represents 30 μm. (C) Western blot of PMN and HUVEC lysates probed with anti-CD157 mAb. (D) Surface expression of CD157 and CD54 (used as a control) was assessed by flow cytometry on HUVECs treated with selected cytokines and chemical mediators for the times indicated. Number of events was 10 000. The difference between control and treated cells was calculated as: fold difference = treated cells (MFI [anti-CD157 or -CD54] – MFI negative control)/(MFI [anti-CD157 or -CD54] – MFI negative control) in the untreated cells. Results are expressed as mean ± SD of 3 experiments. *P < .05, **P < .001.
Analysis of CD157 expression. (A) Flow cytometric analysis of the surface expression of CD157, CD54, and CD31 on HUVECs. Gray-filled profiles are the isotype-control mAb. Number of events was 10 000. Results are representative of 5 experiments. (B) Confluent HUVEC monolayers were stained and the expression of CD157, CD54, and CD31 analyzed by laser-scanning confocal microscopy. Cells were imaged using a ×60/1.4 numerical aperture (NA) oil immersion objective. Bar represents 30 μm. (C) Western blot of PMN and HUVEC lysates probed with anti-CD157 mAb. (D) Surface expression of CD157 and CD54 (used as a control) was assessed by flow cytometry on HUVECs treated with selected cytokines and chemical mediators for the times indicated. Number of events was 10 000. The difference between control and treated cells was calculated as: fold difference = treated cells (MFI [anti-CD157 or -CD54] – MFI negative control)/(MFI [anti-CD157 or -CD54] – MFI negative control) in the untreated cells. Results are expressed as mean ± SD of 3 experiments. *P < .05, **P < .001.
CD157 is involved in transendothelial migration of neutrophils
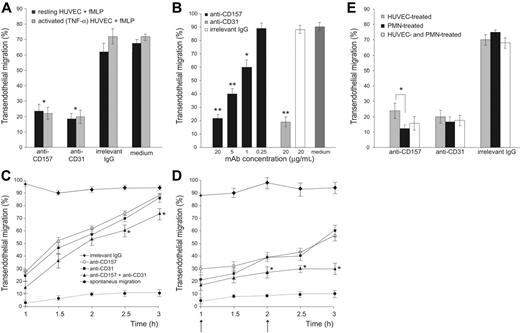
The role of CD157 in fMLP-induced transendothelial migration was investigated by conventional in vitro assays under static conditions. An anti-CD31 mAb was used as a positive control for blocking transmigration.26,27 An isotype-matched anti-HLA class I mAb was used as a negative control to rule out the possibility that the mere binding of a cell surface molecule by murine IgG might perturb the interactions between neutrophils and HUVECs. The potential interference of Fc receptor-mediated effects was prevented by pretreating PMNs with saturating concentrations of murine Fc fragments.28
Anti-CD157 mAb significantly inhibited (> 65%) diapedesis of neutrophils, with an efficiency comparable to that mediated by anti-CD31 (Figure 2A). The inhibitory effect of anti-CD157 mAb was dose-dependent, with significant effects down to 1 μg/mL. All the mAbs used (RF-3, Bec-7, SG-2, and Mo5) showed the same inhibitory effects because they recognized the same or very close epitopes, as inferred by binding-competition experiments.17 The anti-HLA class I mAb had no effects, even at 20 μg/mL (Figure 2B). The results indicate that CD157 is involved in diapedesis of neutrophils across resting HUVEC monolayers in response to fMLP.
The function of CD157 was successively analyzed in the same context but using TNF-α–activated HUVEC monolayers, thereby simulating an inflammatory microenvironment characterized by accelerated neutrophil diapedesis. The inhibition of migration observed after CD157 ligation confirmed that the molecule plays the same role seen when using resting HUVECs across which neutrophils migrate in response to basal concentrations of chemokines (Figure 2A).
The inhibitory effect exerted by anti-CD157 mAb was reversible; indeed, the inhibition was appreciable as long as the CD157 molecule was bound. Similar results were observed using anti-CD31 mAbs. However, neutrophils reacquired the ability to transmigrate when the excess of mAb was removed and the surface-bound mAb was internalized or shed (Figure 2C). Inhibition of transmigration remained constant when the mAbs were refilled during the time course of the experiments. Simultaneous mAb binding of CD157 and CD31 molecules showed an additive inhibitory effect, suggesting that CD157 and CD31 control different steps in the transmigration process (Figure 2D).
The next step was to determine whether the CD157 expressed by neutrophils and by HUVECs contributed differently to transmigration. Experiments were performed by incubating neutrophils or alternatively HUVECs with anti-CD157 mAb and washing away the unbound mAb before transmigration. The results indicated that ligation of CD157 expressed either by neutrophils or by endothelial cells strongly inhibited transmigration; however, CD157 ligation on neutrophils was more efficient (Figure 2E).
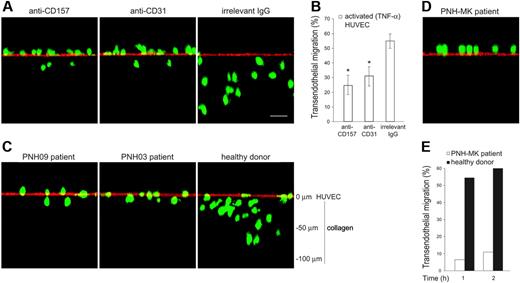
To understand to what extent the inhibitory effects of CD157 ligation on neutrophil transmigration were attributable to suppression of fMLP-induced chemotaxis,17 the contribution of CD157 was evaluated in TNF-α–induced transendothelial migration, in the absence of fMLP. The role of CD157 during neutrophil diapedesis was examined by confocal microscopy of neutrophils migrating across HUVECs grown on collagen29 and by conventional transendothelial migration assays. Cross-sections of the endothelial monolayer, analyzed in the z-plane, showed that in the presence of anti-CD157 mAb, neutrophils remain on the apical surface of the endothelium, with only a few cells crossing the HUVEC monolayer and mostly into the shallow portion of the collagen. Similar effects were observed using neutrophils treated with anti-CD31 mAb.30 In the presence of an anti-HLA class I mAb (used as a class-matched binding mAb), neutrophils migrate through the activated HUVEC monolayer, with most cells up at different depths of collagen, as expected (Figure 4A). Conventional transmigration assays demonstrated that ligation of CD157 significantly inhibited the TNF-α–induced diapedesis with an efficiency comparable to that mediated by ligation of CD31 (Figure 4B). Altogether, these results strongly suggest that CD157 takes part in the diapedesis process.
Transendothelial migration of neutrophils from patients with PNH
Patients with PNH were used as a natural knock-out model to evaluate the functional role of CD157.
CD157 is involved in neutrophil transendothelial migration. PMNs (106/mL) pretreated with purified murine IgG Fc fragments (150 μg/mL) to block Fc receptor were seeded on HUVEC monolayers grown on fibronectin-coated transwell filters. Migration is shown in the presence of a 10-nM fMLP transwell gradient. (A) Anti-CD157 mAb (20 μg/mL) significantly blocked transendothelial migration of PMNs. After 1 hour of incubation at 37°C, the transmigrated cells were counted. Results are expressed as the percentage of transmigrated cells. There was no significant difference in the ability of CD157 or CD31 ligation to block transmigration across resting(▪) or TNF-α activated (▦) HUVEC monolayers. *P < .001 anti-CD157 and anti-CD31 versus no antibody or irrelevant IgG. Data are the mean ± SD of 5 independent experiments. (B) Transmigration assays across TNF-α–activated HUVEC monolayers were run for 1 hour in the presence of decreasing concentrations of anti-CD157 mAb, 20 μg/mL anti-CD31 mAb (positive control), or anti-HLA class I mAb (negative control). Data are the mean ± SD of 3 independent experiments. **P < .001 anti-CD157 or anti-CD31 mAb (20 μg/mL) versus no mAb or irrelevant IgG (20 μg/mL). **P < .001 anti-CD157 mAb (5 μg/mL). *P < .05 anti-CD157 mAb (1 μg/mL). (C-D) Anti-CD157 mAb blocks transmigration reversibly. (C) PMNs and HUVECs were treated with 20 μg/mL of the mAb indicated (15 minutes at 20°C), then the mAb was washed away. Transmigration assays were run and the percentage of transmigrated cells was evaluated every 30 minutes for 3 hours. (D) Experiments were run as in panel C, with mAb refilled during the time course, as indicated (arrow). Data are the mean ± SD (n = 3) of a representative experiment; similar results were observed in 3 separate experiments. *P < .05 anti-CD157 mAb along with anti-CD31 mAb versus anti-CD157 or anti-CD31 alone. (E) PMNs and HUVECs use CD157 for transmigration. PMNs and HUVECs were incubated separately with 20 μg/mL anti-CD157 or anti-CD31 mAb, then unbound mAb was washed away. The percentage of transmigrated PMNs was evaluated after 1 hour. Data are the mean ± SD of 4 independent experiments. Ligation of CD157 expressed either by PMNs or by HUVECs strongly inhibits transmigration; however, ligation of CD157 on neutrophils is more efficient. *P < .05 versus ligation of CD157 on HUVECs. Reduction of transmigration using any methods of blocking of CD157 or CD31 was always significant at P < .001 versus irrelevant IgG.
CD157 is involved in neutrophil transendothelial migration. PMNs (106/mL) pretreated with purified murine IgG Fc fragments (150 μg/mL) to block Fc receptor were seeded on HUVEC monolayers grown on fibronectin-coated transwell filters. Migration is shown in the presence of a 10-nM fMLP transwell gradient. (A) Anti-CD157 mAb (20 μg/mL) significantly blocked transendothelial migration of PMNs. After 1 hour of incubation at 37°C, the transmigrated cells were counted. Results are expressed as the percentage of transmigrated cells. There was no significant difference in the ability of CD157 or CD31 ligation to block transmigration across resting(▪) or TNF-α activated (▦) HUVEC monolayers. *P < .001 anti-CD157 and anti-CD31 versus no antibody or irrelevant IgG. Data are the mean ± SD of 5 independent experiments. (B) Transmigration assays across TNF-α–activated HUVEC monolayers were run for 1 hour in the presence of decreasing concentrations of anti-CD157 mAb, 20 μg/mL anti-CD31 mAb (positive control), or anti-HLA class I mAb (negative control). Data are the mean ± SD of 3 independent experiments. **P < .001 anti-CD157 or anti-CD31 mAb (20 μg/mL) versus no mAb or irrelevant IgG (20 μg/mL). **P < .001 anti-CD157 mAb (5 μg/mL). *P < .05 anti-CD157 mAb (1 μg/mL). (C-D) Anti-CD157 mAb blocks transmigration reversibly. (C) PMNs and HUVECs were treated with 20 μg/mL of the mAb indicated (15 minutes at 20°C), then the mAb was washed away. Transmigration assays were run and the percentage of transmigrated cells was evaluated every 30 minutes for 3 hours. (D) Experiments were run as in panel C, with mAb refilled during the time course, as indicated (arrow). Data are the mean ± SD (n = 3) of a representative experiment; similar results were observed in 3 separate experiments. *P < .05 anti-CD157 mAb along with anti-CD31 mAb versus anti-CD157 or anti-CD31 alone. (E) PMNs and HUVECs use CD157 for transmigration. PMNs and HUVECs were incubated separately with 20 μg/mL anti-CD157 or anti-CD31 mAb, then unbound mAb was washed away. The percentage of transmigrated PMNs was evaluated after 1 hour. Data are the mean ± SD of 4 independent experiments. Ligation of CD157 expressed either by PMNs or by HUVECs strongly inhibits transmigration; however, ligation of CD157 on neutrophils is more efficient. *P < .05 versus ligation of CD157 on HUVECs. Reduction of transmigration using any methods of blocking of CD157 or CD31 was always significant at P < .001 versus irrelevant IgG.
PNH is an acquired disorder of hematopoiesis characterized by the presence of clonal blood cells deficient in GPI-linked molecules, and therefore in CD157.31,32 Defective hematopoiesis develops through several lineages, although the majority of granulocytes and monocytes in peripheral blood are GPI defective.33
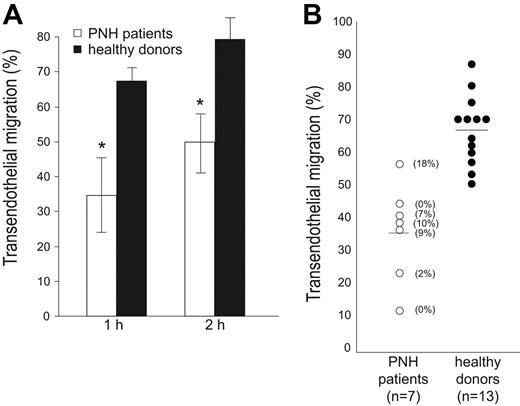
PMNs were obtained from 7 patients with PNH, whose CD157 expression ranged from 0% to 18%; the ability of neutrophils to migrate through endothelial cells was assessed by comparing pathologic cells with normal cells. Tests in pathologic and healthy samples were run in parallel in an in vitro transmigration assay under static conditions. All the experiments were performed in the absence of complement to avoid complement-mediated lysis, critical for patients with PNH who also lack CD55 and CD59, 2 GPI-linked proteins serving as protection against complement-mediated lysis.
The results obtained indicated that neutrophils from PNH display consistently impaired transendothelial migration in the presence of fMLP, as compared with neutrophils from healthy donors, at any time considered (Figure 3A-B). The defect was appreciable both at nanomolar and micromolar concentrations of fMLP (data not shown). A significant interdonor variability in transmigration efficiency is appreciable both in healthy donors as well as in PNH patients, as expected.
The confocal microscopy analysis of neutrophils from PNH patients migrating across HUVECs grown on collagen and activated with TNF-α supported the view of a defect in diapedesis. Indeed, the majority of neutrophils were bound at the apical surface of endothelial cells. Only a reduced number was able to move through the endothelial barrier and to migrate across the subendothelial collagen (Figure 4C). PNH patients, who suffer recurrent infectious diseases, displayed a more severe defect; neutrophils from these PNH patients were almost completely incapable of traversing the TNF-α–activated endothelial barrier both in the absence (Figure 4D) as well as in the presence of fMLP (Figure 4E).
PMNs from patients with PNH exhibit an impaired transendothelial migration. (A) PMNs were seeded on HUVEC monolayers grown on fibronectin-coated transwell filters. Migration is shown in the presence of a 10-nM fMLP transwell gradient. Transmigration assays of PMNs from patients with PNH (□) and from healthy donors (▪) were run in parallel and the percentage of transmigrated cells was evaluated after 1 and 2 hours. Data are expressed as the mean ± SD of 4 separate experiments. *P < .001. (B) Summary of the results of transendothelial migration of neutrophils from 7 patients with PNH (○) analyzed in parallel with 13 healthy donors (•). The percentage of transmigrated cells in the presence of a 10-nM fMLP transwell gradient was evaluated after 1 hour. The number enclosed in parentheses indicates the percentage of CD157+ PMNs. Horizontal bars indicate the mean value of transmigration.
PMNs from patients with PNH exhibit an impaired transendothelial migration. (A) PMNs were seeded on HUVEC monolayers grown on fibronectin-coated transwell filters. Migration is shown in the presence of a 10-nM fMLP transwell gradient. Transmigration assays of PMNs from patients with PNH (□) and from healthy donors (▪) were run in parallel and the percentage of transmigrated cells was evaluated after 1 and 2 hours. Data are expressed as the mean ± SD of 4 separate experiments. *P < .001. (B) Summary of the results of transendothelial migration of neutrophils from 7 patients with PNH (○) analyzed in parallel with 13 healthy donors (•). The percentage of transmigrated cells in the presence of a 10-nM fMLP transwell gradient was evaluated after 1 hour. The number enclosed in parentheses indicates the percentage of CD157+ PMNs. Horizontal bars indicate the mean value of transmigration.
PMNs treated with anti-CD157 or obtained from patients with PNH show reduced transendothelial migration. (A) PMNs from healthy donors were labeled with CFSE, incubated with anti-CD157, anti-CD31, or irrelevant mAb (20 μg/mL), and seeded on TNF-α–activated HUVEC monolayers grown on collagen. After 30 minutes of migration, samples were fixed, washed, stained with anti-CD31 mAb and F(ab′)2-GαMIg-Texas red and evaluated by laser-scanning confocal microscopy (bar represents 30 μm). The images show the position of neutrophils at the end of transmigration assays. No quantitative information must be inferred. Cells were imaged using a × 60 oil immersion objective (1.4 NA). Samples were analyzed by sequential scanning of the xy planes recorded along the z-axis (step size: 1 μm). Series of confocal optical xy images were processed using a 3-dimensional reconstruction program (FluoView 300 software) and visualized as orthogonal views. (B) PMNs were seeded on TNF-α–activated HUVEC monolayers grown on fibronectin-coated transwell filters in the presence of the indicated mAb (20 μg/mL). After 1 hour of incubation at 37°C, the transmigrated cells were counted. Results are expressed as the percentage of transmigrated cells. Data are mean ± SD of 6 experiments performed in triplicate. *P < .01 anti-CD157 mAb and anti-CD31 mAb versus irrelevant IgG. (C) CFSE-labeled PMNs from patients with PNH were seeded on activated HUVECs, incubated, and analyzed as in panel A. Representative experiments from 2 PNH patients are shown. Similar results were observed in all the patients analyzed. (D) Transendothelial migration of PMNs from a representative PNH patient with recurrent bacterial infections analyzed by laser-scanning confocal microscopy, and (E) conventional transmigration assay across TNF-α–activated HUVECs monolayers grown on fibronectin-coated transwell filters. Migration is shown in the presence of a 10-nM fMLP transwell gradient. The difference between transmigration of PMNs from a PNH patient versus PMNs from healthy donor was significant, with P < .001 at any time considered.
PMNs treated with anti-CD157 or obtained from patients with PNH show reduced transendothelial migration. (A) PMNs from healthy donors were labeled with CFSE, incubated with anti-CD157, anti-CD31, or irrelevant mAb (20 μg/mL), and seeded on TNF-α–activated HUVEC monolayers grown on collagen. After 30 minutes of migration, samples were fixed, washed, stained with anti-CD31 mAb and F(ab′)2-GαMIg-Texas red and evaluated by laser-scanning confocal microscopy (bar represents 30 μm). The images show the position of neutrophils at the end of transmigration assays. No quantitative information must be inferred. Cells were imaged using a × 60 oil immersion objective (1.4 NA). Samples were analyzed by sequential scanning of the xy planes recorded along the z-axis (step size: 1 μm). Series of confocal optical xy images were processed using a 3-dimensional reconstruction program (FluoView 300 software) and visualized as orthogonal views. (B) PMNs were seeded on TNF-α–activated HUVEC monolayers grown on fibronectin-coated transwell filters in the presence of the indicated mAb (20 μg/mL). After 1 hour of incubation at 37°C, the transmigrated cells were counted. Results are expressed as the percentage of transmigrated cells. Data are mean ± SD of 6 experiments performed in triplicate. *P < .01 anti-CD157 mAb and anti-CD31 mAb versus irrelevant IgG. (C) CFSE-labeled PMNs from patients with PNH were seeded on activated HUVECs, incubated, and analyzed as in panel A. Representative experiments from 2 PNH patients are shown. Similar results were observed in all the patients analyzed. (D) Transendothelial migration of PMNs from a representative PNH patient with recurrent bacterial infections analyzed by laser-scanning confocal microscopy, and (E) conventional transmigration assay across TNF-α–activated HUVECs monolayers grown on fibronectin-coated transwell filters. Migration is shown in the presence of a 10-nM fMLP transwell gradient. The difference between transmigration of PMNs from a PNH patient versus PMNs from healthy donor was significant, with P < .001 at any time considered.
CD157 plays no significant role in adhesion steps
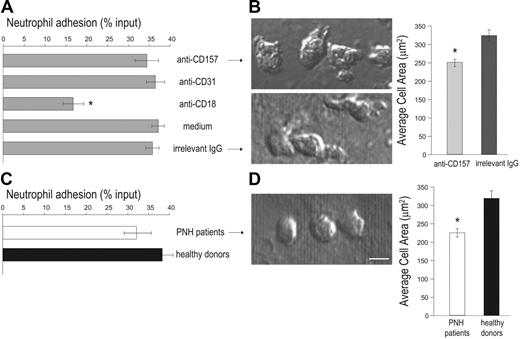
Diapedesis includes multiple concatenated events, so interference at any step invalidates the whole process. Thus, the cascade of events governing transendothelial migration was dissected to identify whether CD157 ligation inhibits tight adhesion (which is a prerequisite for diapedesis), or strictly diapedesis itself. Static adhesion assays were done in the presence of anti-CD157 mAb, using anti-CD18 mAb as a positive control for blocking adhesion.34 CD157 ligation did not affect the number of neutrophils that adhere to HUVEC monolayers stimulated with TNF-α, at any time considered. As expected, blocking CD18 (but not CD31) significantly interfered with neutrophil adhesion to HUVECs (Figure 5A).5,35 This finding demonstrates that, at least in these experimental conditions, CD157 plays no significant role in adhesion efficiency.
Furthermore, the effects mediated by CD157 ligation during interaction between neutrophils and HUVECs were examined at morphologic levels. Treatment of HUVECs with TNF-α significantly increases adhesion of neutrophils, which become elongated, extend lamellipodia (characteristic of a migratory phenotype), and spread. In these conditions, anti-CD157–treated PMNs adherent to the HUVEC monolayer showed a significantly reduced cell area (251.3 ± 7.85 μm2; n = 61) compared to neutrophils treated with an isotype-matched anti-HLA class I mAb (314.79 ± 12.2 μm2; n = 39; Figure 5B). Similar analysis of PMNs obtained from 3 patients with PNH showing severe impairment of diapedesis revealed that these neutrophils adhere to activated HUVECs with the same efficiency as normal neutrophils (Figure 5C) and show a reduced cell area (224.26 ± 8.62 μm2; n = 33) compared to PMN from healthy donors (318.17 ± 12.04 μm2; n = 38; Figure 5D).
Localization of neutrophils on the apical surface of HUVECs
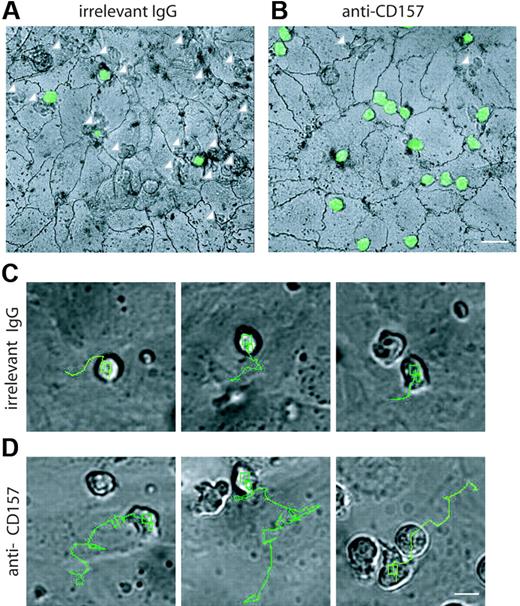
The precise step at which neutrophils are blocked during transendothelial migration was pinpointed by silver staining the junctions at the end of the process. After 1 hour, most of neutrophils were found below the endothelial monolayer (Figure 6A arrows; Table S1), whereas about 20% of cells were on the apical surface. CD157 binding left about 80% of neutrophils on the apical surface (Figure 6B), mainly accumulated at endothelial junctions and at tricellular corners, where the borders of 3 endothelial cells intersect.24 The number of anti-CD157–treated neutrophils located away from the junctions is comparable to that of untreated controls and likely represents the nonmigrating population.36 A small number of cells eluded the mAb effects and crossed the endothelial layer (Figure 6B arrows). These observations further support the conclusion that CD157 does not steer neutrophil movement to the endothelial junctions, similar to CD31 (Table S1).
Analysis of neutrophil movement during extravasation
Real-time microscopy was chosen to investigate the effects of CD157 ligation on neutrophil extravasation. To this aim, HUVEC monolayers were grown on collagen and treated with TNF-α for 4 to 8 hours to prime shear-independent neutrophil diapedesis.22 Sequential images were collected every 10 seconds throughout neutrophil migration. During a 40-minute observation time, the control neutrophils (ie, untreated or treated with an isotype-matched mAb) showed conventional polarization, with filopodia at the leading edge and a uropod at the rear of the cells. The results obtained indicated that neutrophils follow an almost constant route, with movement of about 25 μm in the direction of a nearby junction of interacting HUVECs, later directly crossed (Figures 6C and S2). Crossing the endothelial cell junctions renders the cells phase dark (Video S1). The same experiments performed using neutrophils preincubated with anti-CD157 mAb and activated HUVEC monolayers provided surprising results. Indeed, neutrophils were able to adhere, polarize, and move. However, they migrate a longer distance, sometimes passing by several junctions without crossing them. Only a limited number of neutrophils escape the effects of anti-CD157 mAb ligation and eventually cross a junction after a long journey (∼60 μm; Figure 6D; Video S2). Neutrophils treated with anti-CD31 moved directly to a neighboring junction but were unable to penetrate (Figure S2).
PMNs treated with anti-CD157 or obtained from patients with PNH show efficient adhesion to HUVECs.51Cr-labeled PMNs from healthy donors (A) or PNH patients (C) were plated on activated HUVEC monolayers (in triplicate samples) in the presence or absence of 20 μg/mL of the indicated mAb and incubated at 37°C for 1 hour. After washing, adherent neutrophils were lysed with 2% Triton X-100 (100 μL) and the released radioactivity quantified in a γ counter. Percent specific adhesion was calculated following the formula: (51Cr released from adherent cells/total 51Cr added to each well) × 100. Results represent the mean ± SD of 3 independent experiments. *P < .001 versus all others. Comparison of cell spreading area between neutrophils treated with anti-CD157 or irrelevant IgG (B), or between neutrophils from PNH patients and healthy donors (D). PMNs were plated on HUVEC monolayers treated as described in Figure 4A-D. After 30 minutes, images were acquired by DIC using a × 60 oil immersion objective (1.4 NA; bar represents 10 μm) and analyzed by CellR Imaging software. Data are mean ± SEM of cells analyzed in 3 to 5 experiments. *P < .001.
PMNs treated with anti-CD157 or obtained from patients with PNH show efficient adhesion to HUVECs.51Cr-labeled PMNs from healthy donors (A) or PNH patients (C) were plated on activated HUVEC monolayers (in triplicate samples) in the presence or absence of 20 μg/mL of the indicated mAb and incubated at 37°C for 1 hour. After washing, adherent neutrophils were lysed with 2% Triton X-100 (100 μL) and the released radioactivity quantified in a γ counter. Percent specific adhesion was calculated following the formula: (51Cr released from adherent cells/total 51Cr added to each well) × 100. Results represent the mean ± SD of 3 independent experiments. *P < .001 versus all others. Comparison of cell spreading area between neutrophils treated with anti-CD157 or irrelevant IgG (B), or between neutrophils from PNH patients and healthy donors (D). PMNs were plated on HUVEC monolayers treated as described in Figure 4A-D. After 30 minutes, images were acquired by DIC using a × 60 oil immersion objective (1.4 NA; bar represents 10 μm) and analyzed by CellR Imaging software. Data are mean ± SEM of cells analyzed in 3 to 5 experiments. *P < .001.
Neutrophils treated with anti-CD18 mAb were able to polarize, did not spread on the HUVEC monolayer, remained on the apical surface, and wavered between several different directions as if unable to follow a steady route (Video S3).
Discussion
Leukocyte recruitment to sites of inflammation is a highly controlled process governed by the coordinated interplay of distinct adhesion and signaling molecules.37 The classical molecular interactions driven by adhesion molecules belonging to different families9 are backed by the contribution of other molecules, including several GPI-anchored proteins.38 The present study indicates that CD157 (a member of the NADase/ADP-ribosyl cyclase ectoenzymes family) plays a role in neutrophil extravasation; indeed, it is crucial for their migration through endothelial junctions. Several observations support these conclusions. First, during diapedesis, anti-CD157 mAb binding blocks neutrophils on the apical surface of the endothelium close to a junction, without affecting the number of PMNs that adhere to HUVECs. In this respect, CD157 differs from CD55, another GPI-anchored molecule involved in neutrophil transmigration, that regulates neutrophil tight adhesion, which is a prerequisite to diapedesis but not diapedesis itself.38 Neutrophils treated with anti-CD157 appear to lose their meandering, showing a prolonged and disoriented motility over the endothelial surface; they cross over several endothelial junctions without entering. This effect is independent from nonspecific Fc receptor binding because (1) it is reproducible even after Fc receptor blocking, and (2) an isotype-matched control mAb able to bind both HUVECs and neutrophils has no effects. CD157 expressed by endothelial cells is mainly localized at cell-to-cell contacts and shows a patchy expression profile characteristic of many GPI-linked molecules segregating in cholesterol-rich membrane microdomains.39 Its constitutive expression is not modified by proinflammatory cytokines or other chemical mediators. These findings support the hypothesis that CD157 takes part in the early events of diapedesis. A plausible interpretation might be that CD157 contributes to “educate” the migrating neutrophil and possibly its endothelial cell counterpart to properly rearrange and to initiate productive diapedesis.17,40 Second, combined ligation of CD157 and CD31 results in an additive inhibitory effect, suggesting that the 2 molecules control sequential steps that are both required for an optimal diapedesis. Finally, CD157– neutrophils derived from patients with PNH are characterized by a defective or severely impaired transendothelial migration. The experimental findings mediated by CD157 mAb ligation mirror the scenario observed on neutrophils obtained from patients with PNH. Indeed, CD157-blocked neutrophils and neutrophils from patients with PNH display multiple functional similarities, including reduced chemotaxis17 and impaired migration through endothelial cells and collagen along with efficient adhesion to vascular endothelium. These findings show that the engagement of CD157 in vitro by appropriate mAbs on normal neutrophils leads to the same functional consequences as its absence in vivo secondary to a somatic mutation on neutrophils from patients with PNH, thus highlighting the role of CD157 in diapedesis. However, a variety of GPI-anchored proteins expressed by normal neutrophils are missing on PNH neutrophils; therefore, the possibility that GPI-anchored molecules other than CD157 may contribute to the functional defects cannot be completely ruled out.
Localization of PMNs on the apical surface of HUVECs. PMNs from healthy donors were labeled with CFSE, incubated with anti-CD157 or anti-HLA class I mAb, and seeded on TNF-α–activated HUVEC monolayers grown on collagen. After 1 hour, samples were stained with silver nitrate to identify endothelial cell junctions. These representative apical views show where neutrophils are found, but are not meant to represent the results of the assays quantitatively. In the presence of the isotype-matched control mAb (A) neutrophils are below the endothelial monolayer (white arrows) and few cells are located on the apical surface of HUVECs. CD157 ligation leaves most of neutrophils on the apical surface (B), mainly accumulated at endothelial cell junctions and at tricellular corners. A limited number of cells crossed the endothelial layer (white arrows). Representative images are shown. Samples were observed simultaneously by DIC and fluorescence confocal microscopy. Cells were imaged using a × 60 oil immersion objective (1.4 NA; bar represents 30 μm). The routes of several transmigrating neutrophils treated with isotype-matched mAb (C) or anti-CD157 (D) are indicated. Control neutrophils reach the junction, stop, and begin to cross it. Neutrophils treated with anti-CD157 mAb move at least twice the distance of control neutrophils before reaching the site of diapedesis. In each experiment, neutrophils were plated on confluent HUVEC monolayers, and frames were captured every 10 seconds over a period of 40 minutes (bar represents 10 μm). Frames were then combined in stacks and exported in AVI format as movies. All the images were processed using the TrackIt! software (Olympus Biosystems). To measure the trajectory length of each migrating neutrophil, the cell centroid was tracked from the moment of cell-to-cell contact to the beginning of diapedesis (Figure S2). Micrographs are representative of 5 independent experiments. For a complete set of images, see Videos S1, S2, and S3 online.
Localization of PMNs on the apical surface of HUVECs. PMNs from healthy donors were labeled with CFSE, incubated with anti-CD157 or anti-HLA class I mAb, and seeded on TNF-α–activated HUVEC monolayers grown on collagen. After 1 hour, samples were stained with silver nitrate to identify endothelial cell junctions. These representative apical views show where neutrophils are found, but are not meant to represent the results of the assays quantitatively. In the presence of the isotype-matched control mAb (A) neutrophils are below the endothelial monolayer (white arrows) and few cells are located on the apical surface of HUVECs. CD157 ligation leaves most of neutrophils on the apical surface (B), mainly accumulated at endothelial cell junctions and at tricellular corners. A limited number of cells crossed the endothelial layer (white arrows). Representative images are shown. Samples were observed simultaneously by DIC and fluorescence confocal microscopy. Cells were imaged using a × 60 oil immersion objective (1.4 NA; bar represents 30 μm). The routes of several transmigrating neutrophils treated with isotype-matched mAb (C) or anti-CD157 (D) are indicated. Control neutrophils reach the junction, stop, and begin to cross it. Neutrophils treated with anti-CD157 mAb move at least twice the distance of control neutrophils before reaching the site of diapedesis. In each experiment, neutrophils were plated on confluent HUVEC monolayers, and frames were captured every 10 seconds over a period of 40 minutes (bar represents 10 μm). Frames were then combined in stacks and exported in AVI format as movies. All the images were processed using the TrackIt! software (Olympus Biosystems). To measure the trajectory length of each migrating neutrophil, the cell centroid was tracked from the moment of cell-to-cell contact to the beginning of diapedesis (Figure S2). Micrographs are representative of 5 independent experiments. For a complete set of images, see Videos S1, S2, and S3 online.
It is reasonable to assume that the enhanced susceptibility to bacterial infections observed in patients with PNH is related to the impairment of neutrophil functions here demonstrated and not merely to the neutropenia frequently associated with the disease. The myeloid compartment of patients with PNH is characterized by other functional defects. Indeed, PNH monocytes undergo impaired differentiation to dendritic cells and display severe defects in delivering accessory signals for T-cell receptor–dependent T-cell proliferation.41
The absence of inhibition of neutrophil adhesion to endothelial cells following ligation of CD157 with specific mAbs and the efficient adhesion of neutrophils from PNH patients suggest that CD157 does not play an obvious role in this interaction. However, the possibility that the redundant involvement of other receptor-ligand pairs hides a role of CD157 in the adhesion process cannot be ruled out. This is especially true given that the assay used would not detect a fluid shear-dependent role of CD157 on rolling or adhesion to the apical surface of endothelial cells.
The choice to perform transmigration assays under static conditions was dictated by our focus on diapedesis, a process that immediately follows firm adhesion and is independent of fluid shear.22,26,42 Although extravasation of neutrophils is known to be slightly faster under flow conditions, the molecules involved in this process are likely the same.36,43
Real-time microscopy was used to dissect distinct molecular events governing neutrophil transendothelial migration. Because of their extreme brevity, these events are difficult to capture under fluid shear conditions. Real-time microscopy was recently used to identify the locomotion of monocytes to endothelial junctions as a novel step in monocyte extravasation, interposed between firm adhesion and transendothelial migration5 and mainly relying on the β2-integrins. This left the question as to whether the locomotion step is also important for neutrophils (known to engage a slightly different integrin repertoire than monocytes) during diapedesis and as to where and how CD157 operates during extravasation of neutrophils through activated endothelium.
The results obtained demonstrate that neutrophils move by locomotion an average of 20 μm before reaching the junction and beginning diapedesis. In the experimental conditions adopted, about 80% of neutrophils reached the nearest junction and transmigrated within few seconds after adhesion. Neutrophils ligated by anti-CD157 mAbs were able to move by locomotion and to reach a junction but it seems that they did not perceive the signal for subsequent diapedesis and, therefore, moved along the junction without squeezing through. Only a small percentage of cells, after traveling a long distance, was able to escape the block and transmigrated. CD18 ligation blocks neutrophil adhesion, a necessary prerequisite for transmigration. The consequence of CD18 block is that the interaction with HUVECs leads PMNs to polarize and to move for a short distance in random directions.
The molecular details that govern the CD157-mediated effects and the natural ligands of the molecule remain unknown and should be addressed in future studies. Recently, Partida-Sanchez et al44 demonstrated that CD38, the other member of the NADase/cADPR family sharing 36% amino acid homology with CD157,16 regulates chemotaxis of dendritic cells and dendritic cell precursors during the inflammatory response, through the production of cADPR, a Ca2+-mobilizing second messenger.45 However, it is unlikely that CD157 could exploit this signal transduction pathway because it is a poor cyclase on PMNs,17 on endothelial cells (E.O., unpublished observation, January 2006) as well as on many other cell lineages analyzed.20,46 Moreover, the ability of CD157 to mobilize intracellular Ca2+ in PMNs is not regulated by the products of the enzymatic functions of the molecule (eg, cADPR and ADPR) because it is unaffected by 8-Br-cADPR, an antagonist of cADPR. More generally, cADPR is not required for human neutrophil migration.17 However, the members of this ectoenzyme family catalyze the production of other molecules, including nicotinic acid adenine dinucleotide phosphate and adenine homodinucleotides.47 The physiologic significance of these molecules in leukocyte trafficking remains to be determined.
That ectoenzymes have a dual nature is an unequivocal assumption. Indeed, a number of their functions seem to be independent of their enzymatic activities. It is feasible that the large extracellular domains of ectoenzymes and their association with other molecules can mediate response without involvement of their catalytic properties. This is the case for CD157, an unconventional receptor that compensates for its structural ineptitude to exert receptor functions by establishing cross-talk with specific molecules, much in the same way as CD38.48,49 CD157 has been shown to associate with β2-integrin on neutrophils17 and to mediate FAK phosphorylation in transfected fibroblasts.50 FAK is at a crossroad of multiple signaling pathways and plays important roles in the assembly of signaling complexes regulating cell adhesion, spreading, and migration. In this view, it is conceivable that CD157 plays a dual role in neutrophil trafficking. First, polarization and cytoskeletal reorganization mediated by CD157 engagement provide a mechanism whereby neutrophils perceive the presence of the chemokines. Second, CD157 expressed at the interendothelial junctions might behave as a “gatekeeper” regulating an early step of diapedesis by interacting with unknown counter-molecules. CD157 exerts receptor function in different cells,50-52 including human neutrophils17 ; hence, a major challenge for the future will be to understand how CD157 works in the regulation of neutrophil trafficking.
Authorship
Contribution: E.O. participated in designing and performing research; E.V.T. performed real-time microscopy analysis; B.F. and L.L. performed confocal microscopy analysis and Western blot; G.G. contributed reagents and analytical tools; R.N. and L.L. centralized the pathologic review; and F.M. and A.F. designed the research and wrote the paper. All authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare that they have no conflicting financial interests.
Prepublished online as Blood First Edition Paper, August 17, 2006; DOI 10.1182/blood-2006-04-017160.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants from the Associazione Italiana Ricerca Cancro (Milano, Italy); the Fondo Investimenti Ricerca di Base and PRIN (Ministero dell'Istruzione, dell'Università e della Ricerca), by the special project “Trapianti e riparazione tissutale” Ministero della Salute (Roma, Italy), and by the special project “Oncologia” Compagnia SanPaolo (Torino, Italy). The Compagnia SanPaolo, the Regione Piemonte, and FIRMS (International Foundation for Researches in Experimental Medicine) provided valuable financial contributions.
E.O. is a student at the Postgraduate School of Medical Genetics and B.F. is in the PhD program “Human Oncology,” University of Torino Medical School, Torino, Italy. E.V.T. is the recipient of a research fellow from the FIRMS, Torino, Italy.
We thank F. Bussolino for critically reading the manuscript, R. F. Todd 3rd for generously supplying the Mo5 mAb, and K. Ishihara and T. Hirano for providing RF3, Bec-7, and SG2 mAbs.
Sincere thanks to the patients with PNH for their generous and enthusiastic participation in this study.

![Figure 1. Analysis of CD157 expression. (A) Flow cytometric analysis of the surface expression of CD157, CD54, and CD31 on HUVECs. Gray-filled profiles are the isotype-control mAb. Number of events was 10 000. Results are representative of 5 experiments. (B) Confluent HUVEC monolayers were stained and the expression of CD157, CD54, and CD31 analyzed by laser-scanning confocal microscopy. Cells were imaged using a ×60/1.4 numerical aperture (NA) oil immersion objective. Bar represents 30 μm. (C) Western blot of PMN and HUVEC lysates probed with anti-CD157 mAb. (D) Surface expression of CD157 and CD54 (used as a control) was assessed by flow cytometry on HUVECs treated with selected cytokines and chemical mediators for the times indicated. Number of events was 10 000. The difference between control and treated cells was calculated as: fold difference = treated cells (MFI [anti-CD157 or -CD54] – MFI negative control)/(MFI [anti-CD157 or -CD54] – MFI negative control) in the untreated cells. Results are expressed as mean ± SD of 3 experiments. *P < .05, **P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/13/10.1182_blood-2006-04-017160/8/m_zh80240605130001.jpeg?Expires=1769121700&Signature=3nSs0wlfZe1tnq8zwgYl1p0PIpKU8NRV8Joy-KVsGqjNDS52TVitEDU5h~OG8sR7kS2hW9LXAi4FzX8ty97RnDUP7iTCdap8R-KGJEHb1ae-4irVjEmVoobcwIkvx3gDDcLwU~aOXG163AHt0MwQLh7vnc9PCom~SjsvxWCLPXshgKcykcwNeYgMrJ7UDoW6MTt41RoVHuXGzC1-EK7xaR-w1q33qLKo3u2BpuzrNz8L8bMmnOqwgXMVhOPuyx0Vmu~qEM0YoS1OXScXFN-qgCRBTY~ElffDlpkWyymzLiiG9aSdbq5D1lrKKAWDIFyC9dhSqQ2gBh53r9h3F4oQLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal