Abstract
Peripheral T-cell lymphomas (PTCLs) are rare and have a dismal prognosis. The most frequent subtype is PTCL, unspecified. Epstein-Barr virus (EBV) has been detected in around 40% of cases, but its prognostic significance is not fully established. Lymph node samples from 110 patients with PTCL, unspecified included in LNH87 and LNH93 trials were available. EBV status was studied by EBV-encoded small RNA in situ hybridization (EBER-ISH). EBER-ISH showed positive cells in 45 (41%) of 110 patients. Pretreatment characteristics were comparable between positive and negative cases, except for male sex (80% versus 60%, respectively, P = .02). Only 50% of patients achieved complete remission with a 5-year event-free survival (EFS) and overall survival (OS) of 21% and 30%, respectively. EBER-ISH positivity was the sole factor linked with worse EFS, with a 5-year probability of 11% for positive patients. In univariate analysis, factors affecting OS were EBER-ISH positivity, high LDH level, and age older than 60 years. In multivariate analysis, EBER-ISH was associated with a worse OS in the elderly population. Time-dependent analysis showed that the negative impact of EBV was essentially seen in the first 2 years following diagnosis. These results warrant further studies regarding pathogenesis and specific treatment approaches for EBV-associated PTCL patients.
Introduction
T-cell lymphomas are rare in Europe and the United States, where they represent around 15% of non-Hodgkin lymphomas.1,2 As we and others have shown, a T-cell immunophenotype (except for anaplastic large cell lymphoma) is an independent predictor of poor prognosis in aggressive lymphomas.1,3,4 The WHO classification of lymphoid tumors recognizes 16 different entities among peripheral (mature) T- and natural killer (NK)–cell neoplasms.5 Among these, the entity peripheral T-cell lymphoma, unspecified (PTCL-U) accounts for about half the cases of PTCL seen in Western countries.3,6-8 PTCL-U forms a heterogeneous group of predominantly nodal (and occasionally extranodal) diseases that do not fit into the definition of any other recognizable subtype of PTCL. Although their histopathological aspect is highly variable, usual morphologic characteristics include pleomorphic cellular composition, and frequently a polymorphous inflammatory background.5 Most cases display a CD4+/CD8– helper T-cell phenotype, less frequent cases showing a cytotoxic CD4–/CD8+/TIA1+/granzyme B+ phenotype, which has been linked by certain authors to a worse outcome.9 Efforts to identify different entities with pathophysiologic and prognostic significance among PTCL-U are ongoing.10,11
Epstein-Barr virus (EBV), the etiologic agent of infectious mononucleosis, has been linked to a wide range of tumors, mainly of B-cell origin.12 Association of PTCLs with EBV has been first described in the late 1980s,13 and has since been found to be present in an important fraction of cases in series from Asia14-19 and from Western countries.20-23 While some entities (angioimmunoblastic T-cell lymphoma24 and extranodal NK/T-cell lymphoma, nasal type25 ) are almost systematically associated with EBV, the frequency and signification of such an association among others is less well defined. In PTCL-U, EBV has been detected in 25% to 58% of cases using various techniques.14,16-23 Among these, EBV-encoded small RNA–in situ hybridization (EBER-ISH) has the best potential to detect EBV in this setting.26
d'Amore et al27 reported that the detection of EBV in T-cell lymphomas by EBER-ISH was associated with the worst outcome in a register-based population including various histologic subtypes. Nevertheless, the prognostic significance of EBV inside each PTCL category was not studied independently. In order to establish the prognostic impact of EBV in the PTCL-U category, we retrospectively investigated a population of patients with nodal presentation entered on the LNH87 and LNH93 trials of the Groupe d'Etude des Lymphomes de l'Adulte (GELA), using EBER-ISH.
Patients, materials, and methods
Patient selection
Patients studied here include all cases of PTCL-U confirmed by pathological review of a lymph node sample, who were registered in 2 consecutive multicenter trials conducted by the GELA (LNH87 and LNH93) between April 1987 and October 1998. This study was voluntarily limited to nodal cases in order to avoid confusion with specific extranodal PTCL entities.5 Diagnosis was based on both morphologic examination of slides from routinely stained, paraffin-embedded tissue and immunophenotyping results. Immunophenotyping was performed with a panel of antibodies including at least CD20/L26 and CD3, using an indirect immunoperoxidase or alkaline phosphatase–antialkaline phosphatase (APAAP) method. Additional immunophenotypic studies were performed on paraffin-embedded or frozen tissue sections as deemed necessary by the central histology review panel. In undiagnosed cases that were either angioimmunoblastic T-cell lymphoma or PTCL-U, immunostainings with follicular dendritic cell markers CNA 42 (Dako, Glostrup, Denmark), CD21 (Dako), or CD23 (Novocastra, Newcastle, United Kingdom) were performed, and none of the cases fulfilled the criteria for angioimmunoblastic T-cell lymphoma. A subgroup of patients among the EBER-ISH–positive group for which sufficient additional material was available (n = 23) was studied for the expression of cytotoxic granule-associated proteins TIA1 (Immunotech, Marseille, France) and/or granzyme B (Novocastra). The Working Formulation28 and the Kiel classification29 were used to classify lymphoma at the time of enrollment. An additional review was conducted by at least 2 pathologists from the GELA, and specimens were then reclassified according to the WHO classification.5
The results of both trials have in large part been published,30-36 and the protocol design is briefly described here.
LNH8 and LNH93 trials
Both LNH87 and LNH93 protocols were randomized comparative trials. The experimental arm was dependent on age and on a number of prognostic factors (bone marrow or central nervous system involvement, ECOG performance status ≥2, involvement of ≥ 2 extranodal sites, and largest tumor diameter ≥ 10 cm in LNH87; and factors of the age-adjusted International Prognostic Index [aaIPI] score in LNH93).37 In both trials, informed consent was provided according to the Declaration of Helsinki. The LNH87 and LNH93 studies were approved by the IRB of Hôpital Saint Louis in Paris, France.
For younger patients, the reference arm was 3 to 4 courses of dose-intense doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (ACVBP) followed by sequential consolidation,38 and the experimental arm consisted of the following: 8 cycles of methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone (mBACOD) in patients with no adverse factor (LNH87-1)30 ; 4 cycles of ACVBP-like with a second randomization between sequential consolidation and high-dose chemotherapy with stem cell rescue for patients younger than 56 years with at least 1 adverse factor (LNH87-2)31 ; 4 alternating induction cycles of teniposide, ifosfamide, mitoxantrone, mitoguazone, and methotrexate (VIM3) and ACVBP, and then maintenance with 4 alternating courses of etoposide, ifosfamide, and mitoxantrone (VIM) and doxorubicin, cyclophosphamide, vindesine, and methotrexate (ACVM) in patients between 56 and 69 years with at least 1 adverse factor (LNH87-3)32 ;3 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) with additional involved field radiotherapy for patients with 0 aaIPI factors (LNH93-1)34 ; 4 cycles of ACVBP-like followed by a modified consolidation for patients with 1 aaIPI factor (LNH93-2); and 4 cycles of ACVBP-like followed by high-dose chemotherapy with stem cell rescue for patients younger than 60 with 2 or more aaIPI factors (LNH93-3).35
For elderly patients, the reference arm of treatment was 6 to 8 courses of cyclophosphamide, doxorubicin, vincristin, and prednisone (CHOP) or a CHOP-like regimen. The experimental arms were as follows: 4 cycles of CHOP with additional involved field radiotherapy for patients with 0 aaIPI factors (LNH93-4); 8 cycles of CHOP for patients younger than 70 years with 1 to 3 aaIPI factors (LNH93-5)36 ; 6 cycles of reduced CHOP for patients 70 years or older and ECOG performance status (PS) less than 2 (LNH87-4 and LNH93-6)33 ; and oral chemotherapy with etoposide, chlorambucil, and prednisone for patients 70 years or older with a PS of 2 or more (LNH93-7).
Pretreatment evaluation and follow-up
All patients underwent physical examination, chest x-ray, computed tomography scans of the chest and abdomen, and bone marrow biopsy before start of therapy. Response to therapy was evaluated by repeating initially abnormal examinations after induction therapy, which usually consisted of 4 cycles of chemotherapy, and at the end of treatment. Responses were defined as complete response (CR), CR unconfirmed (CRu), partial response (PR), and stable or progressive disease according to standardized criteria.39
EBER in situ hybridization, latency profile, and double-staining methods
The fluorescein-conjugated EBV (EBER 1 and 2) oligonucleotides (Dako) complementary to nuclear RNA portions of the EBER 1 and 2 genes were used as previously described.25 Briefly, deparaffinized tissue sections were rehydrated and pretreated with proteinase K, dehydrated, air-dried, and hybridized overnight at 55°C with ready-to-use fluorescein isothiocyanate (FITC)–conjugated EBER oligonucleotides. After stringent washing with 2SSC at 55°C, an APAAP immunohistochemical detection system was used. The visualization of alkaline phosphatase activity was obtained by incubation in a solution containing bromochloroindolylphosphate and nitroblue tetrazolium (Dako) for 2 hours at pH 9.6. The slides were counterstained with methyl green.
In order to explore the EBV latency profile, we performed immunohistochemical stainings using antibodies against LMP-1 (Dako) and EBNA-2 (Dako) in 25 EBER-ISH–positive cases with sufficient available material. One case each of EBV-positive classical Hodgkin lymphoma (LMP-1) and infectious mononucleosis (EBNA-2) were used as positive controls.
To determine the B- or T-cell nature of EBV-infected cells, we performed a double-labeling procedure using immunohistochemistry and EBER-ISH in a selection of 12 cases with available material. In a first step, the immunohistochemical demonstration of CD20 and CD2 antigens involved the EnVision (Dako) system. In the second step, EBER-ISH was performed as described above (first paragraph in this subsection) using a development involving NBT/BCIP (BioRad, Munich, Germany). All steps were performed under RNAse-free conditions. As a result of this procedure, EBV-infected B or T cells became visible by their black-purple nuclei and brown membrane staining.
A semiquantitative, visual method was used to quantify the positivity of EBER-ISH. EBER-ISH–positive cases were classified into 4 categories (Figure 1A): Cases quoted as 1 showed only rare, less than 1% scattered positive cells; those quoted as 2 showing obvious, although scattered (1% to 5%) positive cells; those quoted as 3 showing numerous (5% to 50%) positive cells; and those quoted as 4 showing homogeneous positivity in more than 50% of cells.
Statistical analysis
Patient characteristics and remission rates were compared using the chi-squared test or Fisher exact test, as appropriate. Overall survival (OS) was calculated from the date of randomization to either death from any cause or last follow-up examination. Event-free survival (EFS) was calculated from the date of randomization to disease progression, relapse, death from any cause, or last follow-up.39 Survival curves were estimated using the product-limit method of Kaplan-Meier and compared using the log-rank test.40 Cox proportional-hazards regression analysis with survival as the dependent variable was used to adjust the effects of the EBER status for potential independent prognostic factors.41 The threshold upon which the EBER expression level showed an impact on survival was checked by including cubic smoothing splines in the risk function of the Cox model.42 Differences between the results of comparative tests were considered significant at a 2-sided P value below .05. All statistical analyses were performed using the Statistical Application System software (SAS, version 9; SAS Institute, Cary, NC).
Estimation of EBER expression level with spline curve. (A) Four-level semiquantitative estimation of different levels of EBER expression, quoted from 0 (EBER-ISH negative) to 4. Examples are shown at original magnification ×100 (top row) and ×200 (bottom row). Images were captured with a Zeiss Axioskop2 microscope (Zeiss, Oberkochen, Germany) and Neofluar 100×/0.1 NA optical lenses (Zeiss). Photographs were taken with a DP70 Olympus camera (Olympus, Tokyo, Japan). Image acquisition was performed with Olympus DP Controller 2002, and images were processed with Adobe Photoshop v7.0 (Adobe Systems, San Jose, CA). (B) The spline curve shows how the relative risk of death changes with the level of EBER positivity. The dotted lines show the 95% confidence interval. Bold lines show the best cut-off point.
Estimation of EBER expression level with spline curve. (A) Four-level semiquantitative estimation of different levels of EBER expression, quoted from 0 (EBER-ISH negative) to 4. Examples are shown at original magnification ×100 (top row) and ×200 (bottom row). Images were captured with a Zeiss Axioskop2 microscope (Zeiss, Oberkochen, Germany) and Neofluar 100×/0.1 NA optical lenses (Zeiss). Photographs were taken with a DP70 Olympus camera (Olympus, Tokyo, Japan). Image acquisition was performed with Olympus DP Controller 2002, and images were processed with Adobe Photoshop v7.0 (Adobe Systems, San Jose, CA). (B) The spline curve shows how the relative risk of death changes with the level of EBER positivity. The dotted lines show the 95% confidence interval. Bold lines show the best cut-off point.
Results
Histologic review of patients included in LNH93 and LNH98 revealed 147 cases with PTCL-U on a lymph node biopsy. By definition, angioimmunoblastic T-cell lymphoma, anaplastic large cell lymphoma, and recognized extranodal entities of the WHO classification were excluded. Slides from paraffin-embedded tissue were available for analysis by in situ hybridization in 110 (75%) of these patients, who form the basis of this study. Baseline characteristics and treatment outcomes were not significantly different between these 110 patients and the 37 patients for whom no material was available for analysis (data not shown).
Baseline characteristics and EBER-ISH results
Baseline patient characteristics are shown in Table 1. The median age was 60 years, and we observed a male predominance (68%), frequent advanced stage (Ann Arbor III-IV) disease (84%), and bone marrow involvement in a third of patients (36%).
Patient characteristics according to EBER-ISH results
. | Study population,*no. (%) . | EBER-ISH negative, no. (%) . | EBER-ISH positive, no. (%) . | P . |
|---|---|---|---|---|
| Treatment protocol | .28 | |||
| LNH87 | 52 (47) | 28 (43) | 24 (53) | |
| LNH93 | 58 (53) | 37 (57) | 21 (47) | |
| Age | .17 | |||
| 60 y or younger | 55 (50) | 33 (50) | 22 (49) | |
| Older than 60 y | 55 (50) | 32 (50) | 23 (51) | |
| Sex | .02 | |||
| Male | 75 (68) | 39 (60) | 36 (80) | |
| Female | 35 (32) | 26 (40) | 9 (20) | |
| LDH | .20 | |||
| Equal or less than N | 42 (42) | 27 (47) | 15 (31) | |
| Greater than N | 60 (58) | 31 (53) | 29 (69) | |
| Stage | .69 | |||
| I to II | 16 (16) | 10 (17) | 6 (14) | |
| III to IV | 89 (84) | 51 (83) | 38 (86) | |
| PS | .73 | |||
| 0 to 1 | 75 (73) | 43 (75) | 32 (72) | |
| 2 to 4 | 28 (27) | 15 (25) | 13 (28) | |
| Extranodal sites | .37 | |||
| 0 to 1 | 68 (63) | 36 (63) | 32 (72) | |
| 2 or more | 39 (37) | 26 (37) | 13 (28) | |
| IPI risk category | .22 | |||
| Low | 24 (24) | 13 (23) | 11 (25) | |
| Low-intermediate | 32 (32) | 17 (30) | 15 (34) | |
| High-intermediate | 35 (35) | 18 (32) | 17 (39) | |
| High | 9 (9) | 8 (14) | 1 (2) | |
| Bone marrow | .37 | |||
| Normal | 64 (64) | 36 (63) | 28 (74) | |
| Involved | 36 (36) | 22 (37) | 14 (26) | |
| Central nervous system | .64 | |||
| Normal | 105 (95) | 61 (94) | 44 (98) | |
| Involved | 5 (5) | 4 (6) | 1 (2) |
. | Study population,*no. (%) . | EBER-ISH negative, no. (%) . | EBER-ISH positive, no. (%) . | P . |
|---|---|---|---|---|
| Treatment protocol | .28 | |||
| LNH87 | 52 (47) | 28 (43) | 24 (53) | |
| LNH93 | 58 (53) | 37 (57) | 21 (47) | |
| Age | .17 | |||
| 60 y or younger | 55 (50) | 33 (50) | 22 (49) | |
| Older than 60 y | 55 (50) | 32 (50) | 23 (51) | |
| Sex | .02 | |||
| Male | 75 (68) | 39 (60) | 36 (80) | |
| Female | 35 (32) | 26 (40) | 9 (20) | |
| LDH | .20 | |||
| Equal or less than N | 42 (42) | 27 (47) | 15 (31) | |
| Greater than N | 60 (58) | 31 (53) | 29 (69) | |
| Stage | .69 | |||
| I to II | 16 (16) | 10 (17) | 6 (14) | |
| III to IV | 89 (84) | 51 (83) | 38 (86) | |
| PS | .73 | |||
| 0 to 1 | 75 (73) | 43 (75) | 32 (72) | |
| 2 to 4 | 28 (27) | 15 (25) | 13 (28) | |
| Extranodal sites | .37 | |||
| 0 to 1 | 68 (63) | 36 (63) | 32 (72) | |
| 2 or more | 39 (37) | 26 (37) | 13 (28) | |
| IPI risk category | .22 | |||
| Low | 24 (24) | 13 (23) | 11 (25) | |
| Low-intermediate | 32 (32) | 17 (30) | 15 (34) | |
| High-intermediate | 35 (35) | 18 (32) | 17 (39) | |
| High | 9 (9) | 8 (14) | 1 (2) | |
| Bone marrow | .37 | |||
| Normal | 64 (64) | 36 (63) | 28 (74) | |
| Involved | 36 (36) | 22 (37) | 14 (26) | |
| Central nervous system | .64 | |||
| Normal | 105 (95) | 61 (94) | 44 (98) | |
| Involved | 5 (5) | 4 (6) | 1 (2) |
For the entire study population, n = 110; for EBER-ISH-negative population, n = 65; for the EBER-ISH-positive population, n = 45.
N indicates upper limit of normal; PS, Eastern Cooperative Oncology Group performance status.
Some parameters may sum less than 110 due to incomplete data.
EBER-ISH was positive at any level in 45 (41%) of 110 analyzed samples. According to the 4-level semiquantitative estimation illustrated in Figure 1A, 16 (14%) of 110 were quoted as 1; 17 (15%) of 110, as 2; 10 (9%) of 110, as 3; and only 2 (1.8%) of 110, as 4. As shown by the variation of the spline curve (Figure 1B), the best threshold lies between no expression (0) and level 1 expression. It is above this threshold that the spline curve crosses the 0 line, thus indicating a negative impact on survival. Thus, the following results separate only negative (0) and positive (1-4) cases.
Baseline characteristics did not differ significantly according to EBER-ISH results, except for sex. In fact, 36 (80%) of 45 EBER-ISH–positive patients were male, as opposed to only 39 (60%) of 65 EBER-ISH–negative patients (P = .02). Patients were well balanced between the different arms of the LNH87 and LNH93 trials.
Influence of EBER-ISH results on treatment outcome
At the end of induction treatment, a CR or CRu was reached in 50% of patients. The CR/CRu rate did not differ according to EBER-ISH results: 49% in the EBER-ISH–positive group versus 51% in the EBER-ISH–negative group (P = .83). The PR rate was also not significantly different: 15% versus 23% in EBER-ISH–positive and EBER-ISH–negative groups, respectively (P = .3).
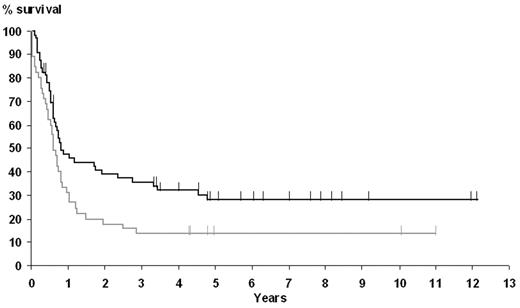
The 5-year OS and EFS rates for the whole population were 30% (95% confidence interval, 21%-39%) and 21% (95% confidence interval, 13%-29%), respectively. Factors affecting OS in univariate analysis were age older than 60 years, LDH level superior to upper limit of normal, an aaIPI score of 2 or more, and EBER-ISH positivity. Five-year OS in the EBER-ISH–positive group was only 20%, as opposed to 34% in the EBER-ISH–negative group (P = .01). Moreover, the presence of EBER-ISH–positive cells was the sole variable associated with a poor EFS (Figure 2), with a 5-year EFS of 11% and 24% in the EBER-ISH–positive and –negative groups, respectively (P = .02).
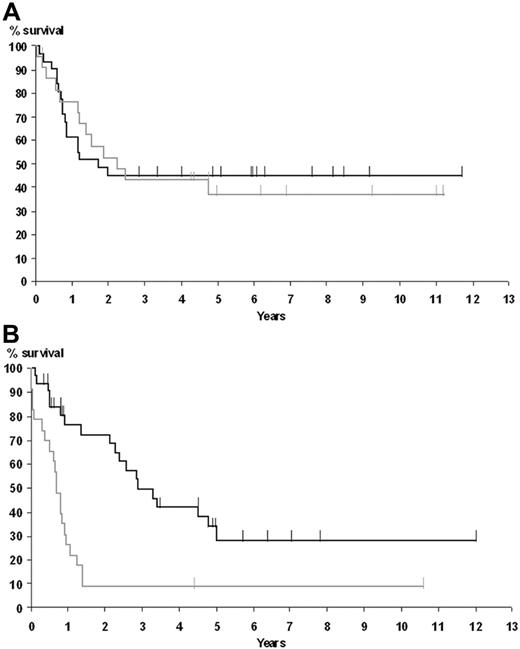
Results of the multivariate analysis of OS (Table 2) showed that an abnormal LDH level (relative risk [RR] = 1.7, P = .001), but not EBER-ISH positivity, was an independent prognostic factor. Furthermore, they showed that EBER-ISH results had a significant interaction with age (P = .001), indicating that its impact on survival varies according to age. Given this result, we plotted the survival estimates according to EBER expression for patient 60 years or younger (Figure 3A) and older than 60 years (Figure 3B). This analysis showed that elderly patients, but not patients younger than 60 years, did worse when EBER-ISH positive. In a multivariate analysis including EBER-ISH status and the aaIPI score, EBER-ISH status was independently associated with survival (RR = 1.77, P = .03). When adding the treatment arm as a variable inside the Cox regression model, no interaction between the EBER-ISH status and treatment arm was observed (data not shown).
Cox regression analysis of overall survival (n = 110)
. | P . | Hazard ratio . |
|---|---|---|
| Age | .37 | 0.81 |
| LDH level | .03 | 1.76 |
| aaIPI | .37 | 0.81 |
| EBER-ISH | .10 | 1.47 |
. | P . | Hazard ratio . |
|---|---|---|
| Age | .37 | 0.81 |
| LDH level | .03 | 1.76 |
| aaIPI | .37 | 0.81 |
| EBER-ISH | .10 | 1.47 |
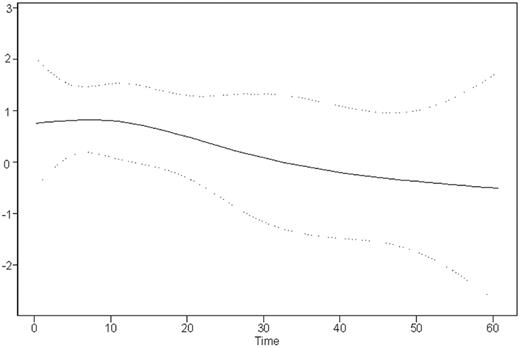
Time-dependent analysis indicates that most of the negative impact of EBER-ISH positivity occurred in the first 2 years after diagnosis: Figure 4 shows the variation of the relative risk (RR) of mortality imparted by EBER-ISH positivity plotted against time (functional form of the EBER-ISH prognostic value).
Histologic, immunophenotypic, and double-labeling studies
In order to explore if some EBER-ISH–positive samples may correspond to cases with borderline morphologic features with other well-known EBV-associated lymphoma types, 36 of 45 EBER-ISH–positive cases with available material were specifically reviewed by 2 of us (J.-F.E., P.G.) in search for subtle histopathological characteristics commonly seen in angioimmunoblastic T-cell lymphoma and extranodal NK/T-cell lymphoma, nasal type. Although all cases were still classified as PTCL-U upon review, 8 cases showed one or more features of angioimmunoblastic lymphoma including vascular expansion (n = 8), clear cell cytology (n = 7), and/or polymorphous inflammatory background with eosinophils and plasma cells (n = 6), but none showed expansion of the dendritic cell network or scattered B blasts. Furthermore, 6 cases showing level 3 or 4 EBER-ISH positivity were found to present some characteristics frequently seen in extranodal NK/T-cell lymphoma: angiocentricity (n = 6) and/or necrosis (n = 5), and 3 of them expressed at least one of the cytotoxic molecules TIA1 or granzyme B. On the whole, 8 of 17 cases investigated for the expression of cytotoxic molecules expressed TIA1, and 2 expressed granzyme B.
Influence of EBER-ISH results on EFS. The curve corresponding to positive patients is shown in gray; negative patients, in black.
Influence of EBER-ISH results on EFS. The curve corresponding to positive patients is shown in gray; negative patients, in black.
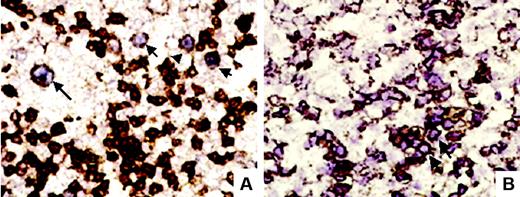
In 12 cases, the cell type infected by EBV was investigated using a double-labeling technique. Of 10 of 12 cases with analyzable results, 6 (all scored as 1 or 2) showed EBV inside CD20+ cells; and 2 (one score 2, one score 3), inside CD2+ cells (examples are shown in Figure 5). In the 2 remaining cases (one score 1, one score 3), EBER-ISH positivity was restricted to cells without demonstrable expression of either CD20 or CD2.
Regarding EBV latency profile, LMP-1 was positive in 13 (52%) of 25 studied cases, and EBNA-2 was positive in only 1 case (4%). This resulted in type I latency profile in 12 cases (48%) and type II latency profile in 12 other cases, whereas only 1 case (4%) showed a type III latency profile.
Overall survival according to age and EBER-ISH results. (A) Patients younger than 60 years. (B) Patients older than 60 years. The curve corresponding to positive patients is shown in gray; negative patients, in black.
Overall survival according to age and EBER-ISH results. (A) Patients younger than 60 years. (B) Patients older than 60 years. The curve corresponding to positive patients is shown in gray; negative patients, in black.
Discussion
PTCL-U represents a heterogeneous subcategory among T-cell lymphomas. Among lymphomas with a helper T-cell phenotype, the expression of various cytokine receptors can be used to distinguish different subcategories with prognostic relevance.10,43 A previous study has suggested a prognostic value for the detection of EBV, which was associated with worst outcome inside a population of PTCLs of various histologic subtypes.27 The goal of our study was to explore the prognostic significance of EBV status within a population composed exclusively of the histopathological category PTCL-U. In order to enhance the homogeneity of the patient population, and to avoid confusion with other well-defined extranodal PTCL entities of the WHO classification, we chose to focus only on cases diagnosed on the basis of a lymph-node biopsy.
The baseline characteristics and treatment outcomes of our patient population were comparable with those of other patient series of PTCL-U.3,6-8 Indeed, we observed a median age around 60 years, a male predominance, and frequent stage III to IV disease, and CR/CRu was achieved in only 50% of cases following chemotherapy. The EBER-ISH technique was found to be positive in 41% of patients, again paralleling the results of previous studies.14,17-20 The high sensitivity of the EBER-ISH technique compared with immunostaining, even in archival material, is well known14,17,18,20 and illustrated here by the fact that LMP-1 was positive in only half of the EBER-ISH–positive cases.
Although the type of treatment varied between protocol arms, all but one patient (109/110) were treated with an anthracyclin-based regimen. All patients 60 years or younger received an intensive regimen with curative intent. Remission rates after therapy did not differ between the EBER-ISH–positive and –negative patients. Univariate analysis of survival nevertheless indicated that EBER-ISH–positive patients had a significantly worse OS and EFS when compared with EBER-ISH–negative patients. Regarding EFS, EBER-ISH positivity was the sole factor (regardless of patient age) associated with a worse survival. Only in the population of patients older than 60 years was this impact still found on multivariable analysis of OS. Treatment intensity was tailored to age in the LNH87 and LNH93 protocols (“Patients, materials, and methods,” under “LNH87 and LNH93 trials”), and elderly patients were on the whole treated less intensively than younger ones. One could suppose that this less-intensive treatment might play a role in the adverse prognostic impact of EBV in elderly patients. Alternatively, EBV-associated PTCLs in elderly patients could represent peculiar diseases associated with age-related immune deficiency, as has been suggested in particular subtypes of EBV-associated B-cell lymphoproliferative disorders arising in elderly subjects.44 The fact that EBV was found inside B cells in some cases may be an indication of underlying immunodeficiency allowing the expansion of EBV-infected nonneoplastic B cells. In agreement with the hypothesis of some local immunodeficiency (in at least some patients), the pattern of EBV infection in half of the studied cases corresponded to type II or, more rarely, type III latency, with EBV-transformed cells expressing LMP-1 antigen, a viral protein known to be a target of cytotoxic T lymphocytes.45
The question whether EBV-associated PTCL-U represents an independent group is open. It is more likely that, despite sharing a bad prognosis, these lymphomas do not form an entity in the clinicobiologic—WHO classification—sense, but rather represent a heterogeneous group of lymphomas having in common the presence of EBV-infected cells. The high variability in the proportion of EBER-ISH–positive cells is an argument favoring this conception. The variable EBV latency profile observed in the 25 studied cases is another argument.
Of interest, some of our EBER-ISH–positive cases showed some morphologic features of angioimmunoblastic T-cell lymphoma or extranodal NK/T-cell lymphoma, nasal type. Furthermore, double-labeling techniques showed that EBV could be found alternatively inside CD20+ or CD2+ cells, further strengthening the variability of the pathogenic process: Most cases (6/10) showed rare EBV-infected B cells, showing a pattern commonly observed in angioimmunoblastic T-cell lymphoma where it is present within the scattered B blasts.24 By contrast, in 2 of 10 cases EBV was found inside CD2+ cells, similar to what is observed in nasal-type NK/T-cell lymphoma, where the virus is found in an episomal form inside a majority of tumor cells.46 In our experience, the prognosis of angioimmunoblastic T-cell lymphoma does not differ with that of PTCL-U.3 Of note, cases resembling nasal-type NK/T-cell lymphoma with an essentially nodal localization and without any extranodal localization have been described.47 Whether they represent true cases of the same disease entity or PTCL-U with expression of cytotoxic molecules as recently described by Asano et al48 is a matter of debate.
Time variation of the relative risk of death between EBER-ISH–positive and –negative patients. The spline curve shows how the relative risk changes over time. Dotted lines show the 95% confidence intervals.
Time variation of the relative risk of death between EBER-ISH–positive and –negative patients. The spline curve shows how the relative risk changes over time. Dotted lines show the 95% confidence intervals.
Double-labeling techniques. (A) EBER-ISH and CD20 double-labeling showed in this case large CD20+ B cells (arrows) showing a purplish nuclear staining corresponding to EBERs (original magnification ×400). (B) In another case, EBER-ISH and CD2 double-labeling showed clusters (arrow) of CD2 and EBER-ISH–positive cells (original magnification ×400).
Double-labeling techniques. (A) EBER-ISH and CD20 double-labeling showed in this case large CD20+ B cells (arrows) showing a purplish nuclear staining corresponding to EBERs (original magnification ×400). (B) In another case, EBER-ISH and CD2 double-labeling showed clusters (arrow) of CD2 and EBER-ISH–positive cells (original magnification ×400).
How EBV plays a role in the pathogenesis of such tumors is not well understood, although recent studies have suggested an oncogenic role for EBERs through the induction of cytokine secretion.49 The fact that only a proportion of cells seems to be infected by EBV in the majority of cases supports the idea that this association is most probably the consequence of a secondary molecular event. Since EBER-ISH positivity was correlated to male sex, a permissive genetic background may also intervene. Of note, some forms of hereditary predisposition to fatal EBV infection are X-linked.50
The prognostic value of EBER-ISH should be explored in future prospective trials. It may help to identify subgroups among PTCL-Us, to guide patient risk stratification and choice of more experimental treatments. As far as possible, molecular quantification of EBV viral load in the blood, which is of predictive value in EBV-associated lymphoproliferations, and study of EBV serologic profiles should be added to such explorations.51
Authorship
Contribution: J.D., N.M., C.G., and P.G. wrote the paper; N.M.-G. performed the experiments; J.-F.E., T.P., F.B., and P.G. performed the pathology review; J.D., N.M., C.G., J.-F.E., and P.G. analyzed the data; and C.G., R.B., A.D., B.C., and F.R. participated in designing the LNH87 and LNH93 studies, and treating and documenting patients. J.-F.E. and N.M. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Félix Reyes died on August 13, 2006. A list of the members of the Groupe d'Etude des Lymphomes de l'Adulte involved in this study appears as a data supplement to the online version of this article.
Prepublished online as Blood First Edition Paper, August 10, 2006; DOI 10.1182/blood-2006-04-017632.
Presented in part at the 43rd annual meeting of the American Society of Hematology, Orlando, FL, December 10, 2001.52
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grant 7705 from the Association pour la Recherche contre le Cancer (ARC).


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal