Abstract
The myelodysplastic syndromes (MDSs) are collections of heterogeneous hematologic diseases characterized by refractory cytopenias as a result of ineffective hematopoiesis. Development of effective treatments has been impeded by limited insights into any unifying pathogenic pathways. We provide evidence that the p38 MAP kinase is constitutively activated or phosphorylated in MDS bone marrows. Such activation is uniformly observed in varied morphologic subtypes of low-risk MDS and correlates with enhanced apoptosis observed in MDS hematopoietic progenitors. Most importantly, pharmacologic inhibition of p38α by a novel small molecule inhibitor, SCIO-469, decreases apoptosis in MDS CD34+ progenitors and leads to dose-dependant increases in erythroid and myeloid colony formation. Down-regulation of the dominant p38α isoform by siRNA also leads to enhancement of hematopoiesis in MDS bone marrow progenitors in vitro. These data implicate p38 MAPK in the pathobiology of ineffective hematopoiesis in lowrisk MDS and provide a strong rationale for clinical investigation of SCIO-469 in MDS.
Introduction
The myelodysplastic syndromes (MDSs) comprise a spectrum of stem-cell malignancies characterized by cytologic dysplasia and ineffective hematopoiesis.1-3 Although approximately one third of patients may experience progression to acute leukemia, refractory cytopenias are the principal cause of morbidity and mortality. MDS can be divided into low- and high-risk subtypes using the International Prognostic Scoring System (IPSS), based on features such as the number of hematopoietic deficits, the percentage of marrow blasts, and cytogenetic pattern.4 Approximately two thirds of patients present with lower-risk disease (Low and Int-1 IPSS scores) characterized by increased rates of apoptosis in the progenitor and differentiated cell compartments in the marrow.5-8 High intramedullary apoptosis leads to ineffective hematopoiesis and peripheral cytopenias. Higher grade or more advanced disease categories (Int-2 and High IPSS scores) are associated with a significant risk of leukemia transformation with a corresponding lower apoptotic index and higher percentage of marrow blasts.
Cytokines play important roles in the regulation of normal hematopoiesis, and a balance between the actions of hematopoietic growth factors and myelosuppressive factors is required for optimal production of different hematopoietic-cell lineages. Excess production of inhibitory cytokines contributes in part to ineffective hematopoiesis in MDS. Tumor necrosis factor-α (TNFα) has been implicated in the increased stem-cell apoptosis seen in MDS,9,10 and high expression of TNF receptors and TNF mRNA have been reported in MDS bone marrows.11-14 Transforming growth factor-β (TGFβ), interleukin-6 (IL-6), vascular endothelial growth factor (VEGF), and interferon (IFN-γ and -α) are also myelosuppressive, and these proinflammatory cytokines have been found to be elevated in serum of patients with MDS in various studies and are hypothesized to play a role in suppressing hematopoiesis in this disease.9,11,15-17 Because multiple cytokines are involved in promoting abnormal hematopoietic development in MDS, targeting one single cytokine may not yield appreciable clinical benefit. In fact, anti-TNF therapeutic strategies (monoclonal antibodies and TNFR blockers) have only shown minimal efficacy.18-21 Thus, it is imperative to identify common targetable pathways that regulate many different cytokines. Our previous studies have shown that myelosuppressive cytokines such as interferons (IFN-α,-β, and -γ), TGFβ, and TNFα can all activate the p38 mitogen activated protein kinase (MAPK) in primary human hematopoietic progenitors. MAP kinases are an evolutionarily conserved family of enzymes that include Erk1/2, p38, Jnk, and Erk5 kinases.22,23 p38 MAPK is a serine-threonine kinase, originally discovered as a stress-activated kinase, that has now been shown to be involved in controlling cell cycle or regulating apoptosis, with its effects being cell and context specific.24-28
We have previously shown that IFN-α and -β, TGFβ, and TNFα treatments lead to dose-dependent inhibition of both myeloid and erythroid colonies in methylcellulose colony-forming assays performed with normal human hematopoietic progenitors.29,30 Furthermore, we have shown that activation of p38 is required for effective biologic activities of these cytokines on hematopoiesis.29,30 Concomitant treatment of hematopoietic cells with pharmacologic inhibitors of p38 MAPK (SB203580 and SB202190) lead to a reversal of the growth inhibitory effects of these cytokines.30 However, the inactive structure analog SB202474 (control) or inhibitors of the Mek/Erk pathway (PD98059) do not reverse the growth inhibition by these 3 cytokines.1,2 Studies to define the molecular basis of these observations have shown that p38 activation is required for transcriptional activation of IFN-sensitive genes but this appears to be unrelated to the effects on DNA binding of Stat complexes or serine phosphorylation of STATs apparently involving a Stat-independent nuclear mechanism.22,31
Our previous studies also show that p38 MAPK inhibition may have a therapeutic role in acquired aplastic anemia (AA).30 Overproduction of TNFα and IFN-γ has been implicated in the generation of the myelosuppressive state in this disease.32-34 p38 Inhibition can stimulate hematopoietic colony formation in AA by interrupting myelosuppressive cytokine signaling1 ; thus, abnormal activation of the p38 pathway may be playing a role in the pathogenesis of AA. There is evidence that a subset of patients with MDS responds to immunosuppressive therapy and shares some similar characteristics with patients with AA. Moreover, in a recent study it was shown that pryimidyl imidazol compounds (SB203580, SB202190) or a pyrazole aryl urea compound (BIX-01208) enhances hematopoietic colony formation from the bone marrows of a small number of patients with the anemia of chronic disease or myelodysplastic syndromes,35 suggesting that p38 may play a role in the pathogenesis of these syndromes as well. In the present study we directly examined whether p38 is phosphorylated or activated in MDS bone marrows. Our data show that p38 is constitutively activated in the bone marrows of patients with MDS, which is not seen in bone marrows derived from patients with other causes of cytopenias. Our data also show that SCIO-469, a novel clinically relevant p38 inhibitor, can decrease stem-cell apoptosis and stimulate hematopoiesis in primary MDS progenitors.
Materials and methods
Cells lines and reagents
Human CD34+ cells were isolated from bone marrows of healthy subjects and patients, after obtaining their informed consent in accordance with the Declaration of Helsinki, for participation in the study protocol approved by the institutional review boards (IRBs) of UT Southwestern Medical School, the Dallas VA Medical Center, the University of Arizona College of Medicine, and the University of South Florida. A portion of human CD34+ cells were also purchased from Cambrex, MA. Erythroid progenitors at the CFU-E level of differentiation were grown in Iscove modified Dulbecco medium (IMDM) enriched with insulin growth factor (IGF), stem-cell factor (SCF), interleukin 3 (IL-3) and erythropoietin (Epo), all of which were obtained from R&D Systems (Minneapolis, MN) as described in our previous studies.18,19,64,36 MDS1 cell line was derived from a patient with MDS with refractory anemia with excess blasts (RAEB) subtype and was provided by Dr Alan List. Human recombinant TNFα was obtained from R&D Systems. p38 Inhibitors SCIO-469 and SD-282 were provided by Scios (Fremont, CA). SCIO-469 has an in vitro IC50 of 9 nM for inhibition of p38α, about 10-fold selectivity for p38α over p38β, and at least 2000-fold selectivity for p38α over an in vitro panel of 20 other kinases, including other MAP kinases. No significant affinity was detected in a panel of 70 enzymes and receptors. SCIO-469 was diluted in DMSO (20 mM stock solution) and kept at –20°C until use. SB203580, SB202190, SB202474, and PD98059 were purchased from Calbiochem (La Jolla, CA). Antibodies against MapKapK-2 and the phosphorylated forms of p38 and MapKapK-2 were obtained from Cell Signaling Technology (Beverly, MA). Antibodies against p38α were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell lysis and immunoblotting
Immunohistochemistry
Paraffin-mounted bone marrow core biopsy sections from patients with MDS and control subjects were obtained after informed consent. Control subjects had anemia from non–MDS-related causes. Slides were deparaffinized and hydrated. Mercury pigments from B5 fixative were removed by iodine-sodium thiosulfate sequences. After rinsing 3 times in PBS, all sections were immersed in 3% hydrogen peroxide for 20 minutes at room temperature to completely block endogenous peroxidases. Antigen retrieval (Citrate Buffer, pH 6.0) was used for all these antibodies. To prevent nonspecific binding with primary antibodies, sections were pretreated with 15% normal goat serum. After 3 washes with PBS, the sections were incubated with primary antibodies overnight at 4°C. The primary antibodies used in this study were rabbit phospho-p38 monoclonal antibody diluted at 1:50 (Cell Signaling Technology), mouse monoclonal CD34 Ab-1 (Lab Vision, Fremont, CA), and affinity-purified rabbit-activated caspase 3 antibody (R&D Systems) diluted at 1:400. After 3 washes with PBS, the sections for caspase 3 and CD34 staining were then incubated with biotinylated goat antirabbit (Chemicon International, Temecula, CA) and goat antimouse (Chemicon International) secondary antibodies, respectively, at 1:2000 dilution at room temperature for 30 minutes. Normal rabbit or mouse IgG (Santa Cruz Biotechnology) was used as negative control. All sections were then treated with ABC reagents (Vector, Burlingame, CA) and finally stained with diaminobenzidine (Research Genetic, Carlsbad, CA). Following several more rinses, the sections were counterstained with hematoxylin and subsequently mounted with Permount mounting medium. The quantification of phospho-p38 and cleaved–caspase-3 staining was analyzed by counting the total number of positively stained cells and by measuring the intensity of the positively stained cells in 5 hot fields (hot field is defined as area of high density of phospho-p38 or caspase 3 staining) for each patient sample under ×400 magnification aided by Image Pro Plus software (Media Cybernetics, Silver Spring, MD). The results were expressed as mean number of positively stained cells per field and mean intensity per field for each individual patient sample.
Flow cytometry
Apoptosis. Primary human bone marrow mononuclear cells were obtained from healthy volunteers after IRB approved informed consent. CD34+ cells were obtained after immunomagnetic selection and were suspended in IMDM in the presence and absence of 20 ng/mL TNFα and 100 nM SCIO-469. Apoptotic cells were evaluated after 24 hours by staining with Annexin V–Alex Fluor 488 dye (BD Bioscience, San Diego, CA). Necrotic cells were visualized in the same assay by staining with nucleic acid dye, Sytox green (Vybrant Apoptosis Kit; Molecular Probes, Carlsbad, CA).
MDS bone marrow mononuclear cells were obtained after IRB informed consent. They were cultured in IMDM with 20% FBS in the presence and absence of 500 nM SCIO-469 for 48 hours. Three MDS samples were evaluated by 3-color flow cytometry after staining them with CD34-APC, Annexin V-FITC, and propidium iodide (PI). Four-color flow cytometry was performed in the next 2 samples using CD34-APC, CD71-PE, Annexin V-FITC, and 7AAD. Apoptosis was evaluated in all samples by determining Annexin V positivity in a gated population of CD34+ cells.
Cell proliferation. Purified primary BM CD34+ progenitors (5 × 105; Stem Cell Technologies, Vancouver, BC) were cultured for 6 days in IMDM with 20% FBS and enriched with TPO, Flt3L, and SCF (all from R&D Systems) with or without 20 ng/mL TNFα and in the presence and absence of 500 nM SCIO-469. Cell cultures were labeled with 10 μM bromodeoxyuridine (BrDU) for the last 16 hours of incubation. Cells were collected, washed with staining buffer, and labeled with anti–CD34-FITC (BD Bioscience). Cells were then fixed, permeabilized, DNAse-treated, and stained with anti–BrDU-APC and 7-AAD using the APC BrDU Flow Kit (BD Bioscience) and analyzed by flow cytometry using LSR 2. BrDU incorporation was evaluated against the amount of 7-AAD staining in a gated population of CD34+ cells.
Immunofluorescence. Bone marrow core biopsy sections from patients with MDS were obtained after IRB informed consent. The biopsies were decalcified by prolonged exposure to EDTA. This was done instead of standard acid decalcification to obtain better signals on immunofluorescence. Paraffin blocks were used to prepare sections. Slides were deparaffinized and hydrated. After rinsing 3 times in PBS, all sections were immersed in 3% hydrogen peroxide for 20 minutes at room temperature to completely block endogenous peroxidases. Antigen retrieval (citrate buffer, pH 6.0) was used for all these antibodies. To prevent nonspecific binding with primary antibodies, sections were pretreated with 15% normal goat serum. Cytonin treatment was used for permeabilization. After 3 washes with PBS, the sections were incubated with rabbit phospho-p38 antibody (Cell Signaling) diluted at 1:50 at 4°C overnight. TACS in situ Apoptosis Detection Kit (R&D Systems; catalog no. TA4627) was used to identify apoptotic cells by detecting DNA fragmentation in bone marrow biopsy. Biotinylated nucleotides are incorporated into the 3-OH ends of the DNA fragments by terminal deoxynucleotidyl transferase (TdT) as per the directions of the kit. The biotinylated nucleotides were detected using a streptavidinfluorescein conjugate. After 3 washes with PBS, the sections were then incubated with goat anti–rabbit IgG Alexa Fluor 568 (catalog no. A11011; Molecular Probes) secondary antibodies at 1:200 dilution at room temperature for 30 minutes. Both secondary antibodies alone and primary antibodies alone were used as negative controls. Following several more rinses, the sections were counterstained with DAPI and subsequently mounted with aqueous mounting medium. Fluorescence was analyzed by Olympus Fluorescent microscope under × 60 magnification.
siRNA transfections
Small interfering RNA duplexes (siRNAs) against p38α were synthesized and purified by Dharmacon (Lafayette, CO). siRNA (100 nM) consisting of a mixture of 4 different RNA duplexes in equimolar concentrations was used for higher knockdown of p38α gene. The target sequences for siRNAs were GAACUGCGGUUACUUAAAC, GCACACAGAUGAUGAAAUG, GGAAUUCAAUGAUGUGUAU, and GAAGCUCUCCAGACCAUUU. The siRNA duplexes were labeled with FITC to show successful transfection in primary CD34+ cells (Label IT siRNA Tracker; Mirus, Madison, WI). CD34+ cells were transfected with either p38α-specific siRNA duplexes or the control scrambled siRNA using the Mirus TKO transfection system (Mirus). Fluorescent microscopy showed a high transfection efficiency of FITC-labeled siRNAs in both normal and MDS-derived CD34 cells (range, 60%-80%). Down-regulation of p38α in primary CD34 cells was assessed 48 hours after transfection by immunoblotting against antibodies for p38α (Santa Cruz Biotechnology). The remainder of the cells were grown in methylcellulose to evaluate for myeloid and erythroid colony formation. The efficiency of anti-p38α siRNAs in inhibiting p38 MAPK RNA (by reverse transcriptase–polymerase chain reaction [RT-PCR]) and protein (by immunoblotting) was also confirmed in a variety of hematopoietic cell lines (data not shown).
Hematopoietic progenitor-cell assays
Hematopoietic progenitor colony formation was determined by clonogenic assays in methylcellulose, as in our previous studies.29,30 All participants in the study signed informed consent, approved by IRB of UT Southwestern and Dallas VA Medical Center. Granulocyte and macrophage colony-forming units (CFU-GMs) and erythroid burst-forming units (BFU-Es) from bone marrow samples were scored on day 14 of culture.
Results
p38 MAPK is constitutively activated in low-grade myelodysplastic syndromes
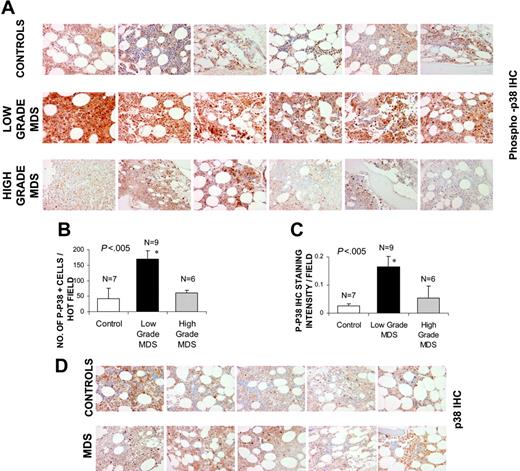
Bone marrows of patients with MDS were assessed for the activation or phosphorylation state of p38 MAPK by immunohistochemistry. Patients were divided into lower (Low and Int-1 IPSS) and higher (Int-2 and High IPSS scores) grade subtypes of MDS (Table 1). MDS bone marrow samples were compared with age-matched controls with non-MDS causes of cytopenias (1 with iron deficiency anemia, 1 with vitamin B12 deficiency, 2 with chemotherapy-related anemia, 2 with chronic renal insufficiency, and 1 with anemia associated with multiple chronic medical problems with high ferritin in the absence of any dysplasia). Notable activation of p38 was seen in bone marrow cells of all patients with low-grade MDS (Figure 1A), with a greater number of phospho-p38–positive staining cells (Figure 1B) and significantly higher intensity of staining (Figure 1C) when compared with controls. Activation of p38 was seen in all subtypes of low-grade MDS examined (1 with refractory anemia [RA], 1 with refractory anemia with ringed sideroblasts [RARS], and 7 with refractory cytopenias with multilineage dysplasia [RCMD]). The level of activation was significantly decreased in high-grade cases and was comparable to controls.
Characteristics of MDS patients
No. . | Age, y/sex . | WBC count, × 109/L . | Hgb level, g/L . | Platelet count, × 109/L . | Cytogenetics . | Subtype . | IPSS score . | IPSS grade . |
|---|---|---|---|---|---|---|---|---|
| 1 | 55/M | 3.4 | 100 | 146 | N | RA | 0 | Low |
| 2 | 81/M | 8.2 | 100 | 239 | N | RA | 0 | Low |
| 3 | 81/M | 3.9 | 78 | 134 | N | RARS | 0 | Low |
| 4 | 86/M | 3.9 | 76 | 130 | -Y | RCMD | 0 | Low |
| 5 | 81/M | 12 | 90 | 131 | N | RCMD | 0 | Low |
| 6 | 76/M | 6 | 70 | 137 | N | RCMD | 0 | Low |
| 7 | 79/M | 6 | 110 | 140 | N | RCMD-RS | 0 | Low |
| 8 | 58/M | 3 | 120 | 106 | N | RA | 0.5 | Int-1 |
| 9 | 71/M | 4.2 | 60 | 130 | N | RCMD | 0.5 | Int-1 |
| 10 | 88/M | 2.4 | 83 | 155 | N | RCMD | 0.5 | Int-1 |
| 11 | 66/M | 5.1 | 110 | 88 | N | RCMD | 0.5 | Int-1 |
| 12 | 56/M | 2 | 90 | 12 | N | RCMD | 0.5 | Int-1 |
| 13 | 77/M | 2 | 120 | 174 | N | RCMD | 0.5 | Int-1 |
| 14 | 81/F | 6.7 | 100 | 155 | -11q | RCMD-RS | 0.5 | Int-1 |
| 15 | 69/F | 4 | 84 | 145 | -7 | RCMD | 1 | Int-1 |
| 16 | 48/F | 5.2 | 84 | 95 | -1q, -11q | RAEB | 1.5 | Int-2 |
| 17 | 61/M | 1.3 | 80 | 19 | N | RAEB | 1.5 | Int-2 |
| 18 | 78/M | 0.6 | 60 | 30 | del 16 (q22) | RCMD | 1.5 | Int-2 |
| 19 | 55/M | 0.3 | 80 | 4 | -20 | RCMD | 1.5 | Int-2 |
No. . | Age, y/sex . | WBC count, × 109/L . | Hgb level, g/L . | Platelet count, × 109/L . | Cytogenetics . | Subtype . | IPSS score . | IPSS grade . |
|---|---|---|---|---|---|---|---|---|
| 1 | 55/M | 3.4 | 100 | 146 | N | RA | 0 | Low |
| 2 | 81/M | 8.2 | 100 | 239 | N | RA | 0 | Low |
| 3 | 81/M | 3.9 | 78 | 134 | N | RARS | 0 | Low |
| 4 | 86/M | 3.9 | 76 | 130 | -Y | RCMD | 0 | Low |
| 5 | 81/M | 12 | 90 | 131 | N | RCMD | 0 | Low |
| 6 | 76/M | 6 | 70 | 137 | N | RCMD | 0 | Low |
| 7 | 79/M | 6 | 110 | 140 | N | RCMD-RS | 0 | Low |
| 8 | 58/M | 3 | 120 | 106 | N | RA | 0.5 | Int-1 |
| 9 | 71/M | 4.2 | 60 | 130 | N | RCMD | 0.5 | Int-1 |
| 10 | 88/M | 2.4 | 83 | 155 | N | RCMD | 0.5 | Int-1 |
| 11 | 66/M | 5.1 | 110 | 88 | N | RCMD | 0.5 | Int-1 |
| 12 | 56/M | 2 | 90 | 12 | N | RCMD | 0.5 | Int-1 |
| 13 | 77/M | 2 | 120 | 174 | N | RCMD | 0.5 | Int-1 |
| 14 | 81/F | 6.7 | 100 | 155 | -11q | RCMD-RS | 0.5 | Int-1 |
| 15 | 69/F | 4 | 84 | 145 | -7 | RCMD | 1 | Int-1 |
| 16 | 48/F | 5.2 | 84 | 95 | -1q, -11q | RAEB | 1.5 | Int-2 |
| 17 | 61/M | 1.3 | 80 | 19 | N | RAEB | 1.5 | Int-2 |
| 18 | 78/M | 0.6 | 60 | 30 | del 16 (q22) | RCMD | 1.5 | Int-2 |
| 19 | 55/M | 0.3 | 80 | 4 | -20 | RCMD | 1.5 | Int-2 |
WBC indicates white blood cell; Hbg, hemoglobin; N, normal cytogenetics; RA, refractory anemia; RARS, refractory anemia with ringed sideroblasts; Y, loss of cytometry; RCMD, refractory cytopenia with multilineage dysplasia; RCMD-RS, refractory cytopenia with multilineage dysplasia and ringed sideroblasts; RAEB, refractory anemia with excess blasts.
p38 MAPK is activated in low-grade MDS. Bone marrow (BM) biopsies from patients with MDS and control subjects with non-MDS causes of cytopenias were fixed and immunostained with antibody against phosphor-p38 MAPK (A). Histologic examination of 6 representative samples of low- and high-grade MDS and controls revealed more intense staining in low-grade MDS samples. The quantification of p-p38 staining was analyzed by counting the total number of positively stained cells (B) and by measuring intensity of the positively stained cells (C) in 5 hot fields (defined as area of high density of p-p38 staining) and aided by Image Pro Plus software. Error bars indicate SEM. Two-tailed t test shows significantly higher p38 activation per hot field in low-grade MDS samples. Differences in total p38 MAPK protein expression in 5 representative samples from each group were also evaluated in MDS and control bone marrows by immunostaining with an antibody against p38 MAPK (D).
p38 MAPK is activated in low-grade MDS. Bone marrow (BM) biopsies from patients with MDS and control subjects with non-MDS causes of cytopenias were fixed and immunostained with antibody against phosphor-p38 MAPK (A). Histologic examination of 6 representative samples of low- and high-grade MDS and controls revealed more intense staining in low-grade MDS samples. The quantification of p-p38 staining was analyzed by counting the total number of positively stained cells (B) and by measuring intensity of the positively stained cells (C) in 5 hot fields (defined as area of high density of p-p38 staining) and aided by Image Pro Plus software. Error bars indicate SEM. Two-tailed t test shows significantly higher p38 activation per hot field in low-grade MDS samples. Differences in total p38 MAPK protein expression in 5 representative samples from each group were also evaluated in MDS and control bone marrows by immunostaining with an antibody against p38 MAPK (D).
Because p38 MAPK is ubiquitously expressed, we also investigated the phenotypes of bone marrow cells that are expressing the activated kinase. Histologic examination revealed that p38 was activated in hematopoietic progenitors of all lineages, including erythroid and myeloid progenitors and even megakaryocytes. Staining with anti-CD3 antibody revealed very few lymphocytes, most of which appeared to be phospho-p38 negative (data not shown). Immunohistochemical staining with an antibody against total p38 MAPK was also performed to determine any changes in p38 MAPK expression in MDS bone marrows when compared with controls. No significant differences in either staining intensity or in the number of p38-positive cells were seen (Figure 1D; Table 2), suggesting that p38 MAPK is overactivated but not overexpressed in low-grade MDS.
Statistical analysis of number of positive cells and intensity of staining
. | Control . | MDS . | P . |
|---|---|---|---|
| p38 Staining intensity/field | 0.18 ± .012 | .0150 ± .0036 | .8 |
| No. p38+ cells/field | 15 ± 6 | 20 ± 4 | .7 |
| Total no. | 5 | 11 | — |
. | Control . | MDS . | P . |
|---|---|---|---|
| p38 Staining intensity/field | 0.18 ± .012 | .0150 ± .0036 | .8 |
| No. p38+ cells/field | 15 ± 6 | 20 ± 4 | .7 |
| Total no. | 5 | 11 | — |
— indicates not applicable.
p38 MAPK activation correlates with enhanced apoptosis in MDS bone marrows
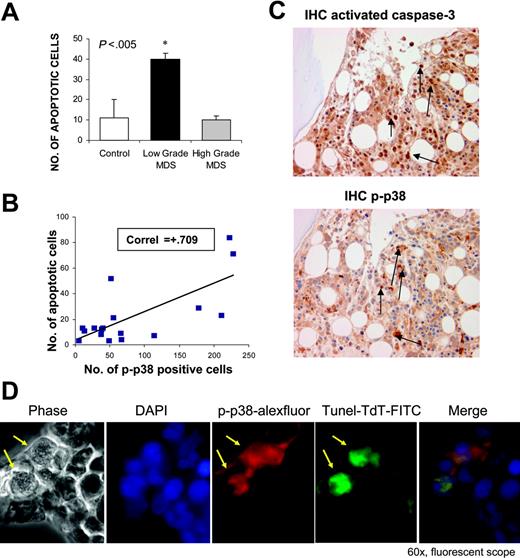
Increased hematopoietic progenitor-cell apoptosis plays a role in the pathobiology of ineffective hematopoiesis in MDS. Because we observed high levels of p38 activation in low-grade MDS, we sought to determine its relation to the degrees of apoptosis seen in this disease. Immunostaining of MDS bone marrows with antibodies against cleaved caspase 3 and phosphorylated-p38 was performed in serial sections of bone biopsies. We observed that cases of low-grade MDS showed a high percentage of cells positive for cleaved caspase 3 (apoptotic cells) consistent with previous studies6 (Figure 2A). These apoptotic cells show strong activation of p38 MAPK in serial sections (Figure 2B-C). There was a positive correlation between p38 activation and apoptosis (Figure 2B), and it appears that p38 MAPK is activated strongly in progenitors that are undergoing apoptosis (Figure 2C). In contrast, the number of apoptotic cells was decreased in high-grade cases and was comparable with controls.
Further determination of this correlation was done by double immunofluorescence staining of MDS bone marrow sections after TdT in situ labeling of apoptotic cells (TUNEL assay) in combination with antibodies against phospho-p38. Merged immunofluorescence showed that cells undergoing apoptosis also displayed high levels of activated p38 (Figure 2D).
SCIO-469 reverses TNF-induced apoptosis of normal hematopoietic progenitors
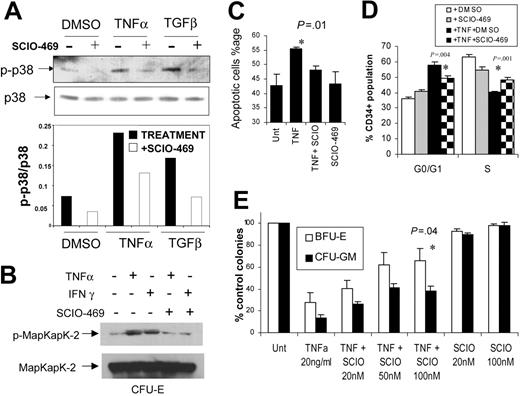
TNFα is a proinflammatory cytokine that has been implicated in the ineffective hematopoiesis seen in MDS. In previous studies we have shown that commercially available p38 inhibitors such as SB203580 reverse the suppressive effects of TNFα on normal hematopoietic progenitors.30 We determined the effects of newer, potent p38 inhibitors on TNFα-induced apoptosis and suppression of normal hematopoiesis. SCIO-469 is a small molecule that acts as an ATP-competitive p38 kinase inhibitor. SCIO-469 selectively inhibits the activity of p38α37,38 and was able to block cytokine-induced phosphorylation of p38 MAPK in MDS1, a cell line derived from a patient with MDS (Figure 3A). Efficacy of SCIO-469 in primary hematopoietic progenitors was shown by its inhibition of phosphorylation and activation of MapKapK-2, a downstream target of activated p38 MAPK (Figure 3B). Primary human BM-derived progenitors at the CFU-E stage of maturation were grown in cytokine-enriched media in the presence or absence of TNFα and with or without SCIO-469.37,38 After 24 hours, TNFα treatment led to increased apoptosis in the progenitors which was inhibited in the presence of SCIO-469 (Figure 3C). The effect of TNF and p38 inhibition on hematopoietic CD34+ cell proliferation was also determined by BrDU incorporation after 6 days of culture. TNF treatment led to a significant G0/G1 arrest with lesser percentage of CD34+ cells in S phase of the cell cycle. This G0/G1 arrest was abrogated in the presence of p38 inhibitor, SCIO-469, showing the role of p38 in TNF-induced myelosuppression (Figure 3D). The cumulative effect on these 2 processes (apoptosis and proliferation) as well as on progenitor differentiation was determined by performing colony assays with TNF in the presence and absence of p38 inhibitors for 14 days. Colony assays with normal CD34+ human hematopoietic cells revealed that TNFα exposure led to a decrease in both erythroid and myeloid colony numbers, and this effect was reversed by SCIO-469 in a dose-dependent manner (Figure 3E).
p38 Activation correlates with apoptosis in low-grade MDS. Bone marrow biopsies from patients with MDS and non-MDS control subjects were fixed and immunostained with antibody against cleaved or activated caspase-3. Number of apoptotic cells (cleaved caspase-3 positive) were determined in cases of low- and high-risk MDS and compared with controls. (A) Cases of low-risk MDS had significantly higher numbers of apoptotic cells (P < .05, 2 tailed t test). The number of apoptotic cells was correlated with numbers of cells positive for phospho-p38. *Statistical significance. Pearson correlation coefficient was calculated with the use of Microsoft Excel (Redman, WA) (B). Serial sections of a representative MDS bone marrow sample show that cells undergoing apoptosis exhibit greater activation of p38 MAPK (C). Bone marrow biopsies from a representative patient with MDS were stained with rabbit anti–human phospho-p38 (red) and fluorescein-TdT (green) after in situ nucleotide labeling for apoptosis detection (TUNEL assay) followed by goat anti–rabbit IgG Alexa Fluor 568 secondary antibodies. Merged immunofluorescence shows apoptotic cells exhibit activated p38 MAPK (positive for phospho-p38) (D). Arrows indicate positive cells.
p38 Activation correlates with apoptosis in low-grade MDS. Bone marrow biopsies from patients with MDS and non-MDS control subjects were fixed and immunostained with antibody against cleaved or activated caspase-3. Number of apoptotic cells (cleaved caspase-3 positive) were determined in cases of low- and high-risk MDS and compared with controls. (A) Cases of low-risk MDS had significantly higher numbers of apoptotic cells (P < .05, 2 tailed t test). The number of apoptotic cells was correlated with numbers of cells positive for phospho-p38. *Statistical significance. Pearson correlation coefficient was calculated with the use of Microsoft Excel (Redman, WA) (B). Serial sections of a representative MDS bone marrow sample show that cells undergoing apoptosis exhibit greater activation of p38 MAPK (C). Bone marrow biopsies from a representative patient with MDS were stained with rabbit anti–human phospho-p38 (red) and fluorescein-TdT (green) after in situ nucleotide labeling for apoptosis detection (TUNEL assay) followed by goat anti–rabbit IgG Alexa Fluor 568 secondary antibodies. Merged immunofluorescence shows apoptotic cells exhibit activated p38 MAPK (positive for phospho-p38) (D). Arrows indicate positive cells.
p38 MAPK inhibition decreases MDS CD34+ progenitor apoptosis
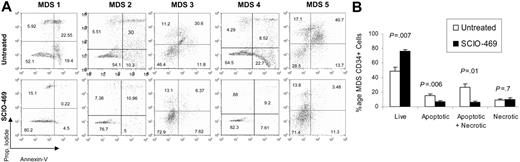
After showing a correlation between MDS progenitor-cell apoptosis and p38 activation, we sought to directly evaluate the functional role of p38 in this phenomenon. Bone marrow mononuclear cells from 5 patients with early or low-grade MDS were cultured in the presence and absence of the selective p38α inhibitor, SCIO-469. BM progenitor apoptosis was determined by Annexin V staining on a gated population of CD34+ cells. SCIO-469 treatment led to significant decrease in the percentage of apoptotic CD34+ cells after 48 hours of culture (P = .006) (Figure 4). Correspondingly, an increase in the number of viable CD34+ cells was also observed in samples treated with the p38 inhibitor (P = .007) (Figure 4).
Down-regulation of p38 MAPK with siRNA promotes hematopoiesis in MDS
To further determine the biologic significance of p38 activation in MDS, siRNAs against p38α were optimally designed and successfully transfected into normal CD34+ hematopoietic stem cells (Figure 5A-B). Using a combination of 4 different siRNA oligos, we were able to achieve significant knockdown of p38α protein expression in these cells after 48 hours (Figure 5A). To determine whether inhibiting p38 can stimulate hematopoiesis in MDS, primary MDS CD34+ cells were successfully transfected with pooled siRNAs against p38α (Figure 5B), and these cells were assessed for hematopoietic colony formation after 14 days. MDS CD34+ cells transfected with anti-p38 siRNAs exhibited a striking increase in both erythroid and myeloid colonies in vitro, pointing to p38 MAPK as a possible therapeutic target for this disease (Figure 5C). To determine whether p38 down-regulation had any effect on progenitor apoptosis seen in MDS, a portion of CD34+ cells transfected with siRNAs were examined 48 hours after transfection by flow cytometry. Annexin V staining revealed that cells transfected with anti-p38 siRNAs had a significantly higher percentage of viable cells with less cells undergoing apoptosis (P = .04, 2-tailed t test) (Figure 5D).
p38 Inhibitor SCIO-469 can reverse TNF-mediated myelosuppression. MDS1 cells were pretreated for 1 hour with vehicle (–) or 1.0 μM SCIO-469 (+) and then induced with either 1 ng/mL TNFα or 5 ng/mL TGFβ for 30 minutes. The p-p38 and total p38 levels were analyzed by Western blotting. Bar graph represents p-p38 levels relative to total p38 in each sample (A). Immunomagnetically selected bone marrow–derived CD34+ cells were differentiated into hematopoietic progenitors at the CFU-E stage of maturation as described before.30 These cells were treated with 20 ng/mL TNFα or 10 000U/mL IFN-γ in the presence and absence of 100 nM SCIO-469. Cell lysates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with an antibody against the phosphorylated form of MapKapK-2 (threonine 334). The same blot was stripped and reprobed with an antibody against total MapKapK-2, to control for protein loading (B). Primary bone marrow–derived CD34+ cells were grown in cytokine-enriched liquid media in the presence and absence of 20 ng/mL TNFα and SCIO-469 (100 nM) for 24 hours. The percentages of apoptotic and dead cells were determined by staining with mixture of Annexin V–Alexa Fluor 488 and nucleic acid dye, Sytox green, respectively (Vybrant Apoptosis Kit; Molecular Probes) (C). Mean of 3 independent experiments showed significant decrease in TNFα-mediated apoptosis in the presence of SCIO-469 (P = .01, paired t test). BM CD34+ progenitors were cultured with TPO, Flt3L, and SCF with or without 20 ng/mL TNFα and in the presence and absence of 500 nM SCIO-469 for 6 days. BrDU incorporation was evaluated against the amount of 7-AAD by flow cytometry to determine the percentage of subpopulation at each cell-cycle stage in a gated population of CD34+ cells. Results from 3 experiments were used to compare the proportion of cells in G0/G1 and S phase of cell cycle by using 2-tailed t test. (D) Primary bone marrow–derived CD34+ cells were cultured in methylcellulose in the presence and absence of 20 ng/mL TNFα and SCIO-469. Colonies were scored on day 14. Results are expressed as mean ± SEM of 3 independent experiments (E). Treatment with SCIO-469 led to a significant reversal of TNF-mediated myelosuppression (P = .04, t test).
p38 Inhibitor SCIO-469 can reverse TNF-mediated myelosuppression. MDS1 cells were pretreated for 1 hour with vehicle (–) or 1.0 μM SCIO-469 (+) and then induced with either 1 ng/mL TNFα or 5 ng/mL TGFβ for 30 minutes. The p-p38 and total p38 levels were analyzed by Western blotting. Bar graph represents p-p38 levels relative to total p38 in each sample (A). Immunomagnetically selected bone marrow–derived CD34+ cells were differentiated into hematopoietic progenitors at the CFU-E stage of maturation as described before.30 These cells were treated with 20 ng/mL TNFα or 10 000U/mL IFN-γ in the presence and absence of 100 nM SCIO-469. Cell lysates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with an antibody against the phosphorylated form of MapKapK-2 (threonine 334). The same blot was stripped and reprobed with an antibody against total MapKapK-2, to control for protein loading (B). Primary bone marrow–derived CD34+ cells were grown in cytokine-enriched liquid media in the presence and absence of 20 ng/mL TNFα and SCIO-469 (100 nM) for 24 hours. The percentages of apoptotic and dead cells were determined by staining with mixture of Annexin V–Alexa Fluor 488 and nucleic acid dye, Sytox green, respectively (Vybrant Apoptosis Kit; Molecular Probes) (C). Mean of 3 independent experiments showed significant decrease in TNFα-mediated apoptosis in the presence of SCIO-469 (P = .01, paired t test). BM CD34+ progenitors were cultured with TPO, Flt3L, and SCF with or without 20 ng/mL TNFα and in the presence and absence of 500 nM SCIO-469 for 6 days. BrDU incorporation was evaluated against the amount of 7-AAD by flow cytometry to determine the percentage of subpopulation at each cell-cycle stage in a gated population of CD34+ cells. Results from 3 experiments were used to compare the proportion of cells in G0/G1 and S phase of cell cycle by using 2-tailed t test. (D) Primary bone marrow–derived CD34+ cells were cultured in methylcellulose in the presence and absence of 20 ng/mL TNFα and SCIO-469. Colonies were scored on day 14. Results are expressed as mean ± SEM of 3 independent experiments (E). Treatment with SCIO-469 led to a significant reversal of TNF-mediated myelosuppression (P = .04, t test).
p38 inhibitor SCIO-469 can decrease apoptosis in MDS CD34+ progenitors. BM mononuclear cells from patients with MDS were cultured in the presence and absence of 500 nM SCIO-469 for 48 hours. Apoptosis in gated population of CD34+ cells was determined by Annexin V staining. Comparison of dot plots from 5 independent experiments shows a decrease in the percentage of Annexin V–positive CD34+ cells in samples treated with SCIO-469 (A). MDS CD34+ progenitors from 5 patients show significantly greater viability and decreased apoptosis after 48 hours of treatment with SCIO-469 (paired 2-tailed t test). Results are presented as means ± SEMs (B).
p38 inhibitor SCIO-469 can decrease apoptosis in MDS CD34+ progenitors. BM mononuclear cells from patients with MDS were cultured in the presence and absence of 500 nM SCIO-469 for 48 hours. Apoptosis in gated population of CD34+ cells was determined by Annexin V staining. Comparison of dot plots from 5 independent experiments shows a decrease in the percentage of Annexin V–positive CD34+ cells in samples treated with SCIO-469 (A). MDS CD34+ progenitors from 5 patients show significantly greater viability and decreased apoptosis after 48 hours of treatment with SCIO-469 (paired 2-tailed t test). Results are presented as means ± SEMs (B).
Treatment with pharmacologic inhibitors of p38 MAPK leads to increased colony formation from MDS hematopoietic progenitors
Having established the potential of p38 inhibition as a therapeutic strategy in MDS, we wanted to determine the efficacy of various small molecule inhibitors of p38 MAPK in this disease. Bone marrow aspirates were collected from 19 patients with a variety of MDS subtypes. Most of the patients had low-grade MDS and did not have increased blast counts (Table 1). Consistent with results seen with siRNAs, treatment with the p38 inhibitor SB203580 (Figure 6) resulted in a striking increase in erythroid (BFU-E) and myeloid (CFU-granulocytic monocytic) colony numbers in all the cases (P < .001). However as expected,35 there were no increases on exposure to MEK inhibitor PD98059 or negative control inactive structural analog SB202474 (Figure 6) (P = .3).
Down-regulation of p38α by siRNA can stimulate hematopoiesis in MDS CD34+ progenitors. A mixture of 4 siRNAs against p38α were transfected in primary CD34+ hematopoietic progenitors using Mirus TKO transfection reagent. Western blotting showed a specific and significant decrease in total p38 protein levels (A). High transfection efficiency was shown by using fluorescent-labeled siRNAs (B). MDS CD34+ cells were transfected with either anti-p38α or control-scrambled siRNAs and grown in vitro in methylcellulose with cytokines. Colonies were scored on day 14, and results were expressed as means ± SEMs of 3 independent experiments. Significantly higher number of both myeloid (CFU-GM) and erythroid (BFU-E) colonies were observed in cells transfected with anti-p38 siRNAs (C). MDS CD34+ cells transfected with anti-p38α and scrambled control siRNAs were evaluated after 48 hours by Annexin V staining. Flow cytometry revealed a significantly higher percentage of viable cells (P = .045, t test) and fewer number of apoptotic cells (P = .04, t test) when transfected with anti-p38α siRNA (D). Results are presented as means ± SEMs of 5 independent experiments.
Down-regulation of p38α by siRNA can stimulate hematopoiesis in MDS CD34+ progenitors. A mixture of 4 siRNAs against p38α were transfected in primary CD34+ hematopoietic progenitors using Mirus TKO transfection reagent. Western blotting showed a specific and significant decrease in total p38 protein levels (A). High transfection efficiency was shown by using fluorescent-labeled siRNAs (B). MDS CD34+ cells were transfected with either anti-p38α or control-scrambled siRNAs and grown in vitro in methylcellulose with cytokines. Colonies were scored on day 14, and results were expressed as means ± SEMs of 3 independent experiments. Significantly higher number of both myeloid (CFU-GM) and erythroid (BFU-E) colonies were observed in cells transfected with anti-p38 siRNAs (C). MDS CD34+ cells transfected with anti-p38α and scrambled control siRNAs were evaluated after 48 hours by Annexin V staining. Flow cytometry revealed a significantly higher percentage of viable cells (P = .045, t test) and fewer number of apoptotic cells (P = .04, t test) when transfected with anti-p38α siRNA (D). Results are presented as means ± SEMs of 5 independent experiments.
Most interestingly, similar results were observed on treatment with 2 new p38 MAPK inhibitors SCIO-469 and SD-282. These compounds are potent, selective inhibitors of p38α and show at least a 10-fold higher affinity for p38α as compared with p38β.37-40 SCIO-469 is an oral compound presently being used in phase 2 clinical trials for rheumatoid arthritis and other diseases. On exposure to these 2 inhibitors, MDS progenitors showed increases in both erythroid and myeloid colony numbers in vitro (Figure 6; P < .001). Consistent with previous studies, untreated MDS CD34+ cells exhibited poor colony-forming ability in vitro, showing poor hematopoietic potential of these cells. Strikingly, treatment with SCIO-469 at low doses (20-100 nM) was able to increase myeloid and erythroid colony numbers 2- to 3-fold in MDS progenitors, pointing to therapeutic potential of SCIO-469 in early or low-grade MDS.
Discussion
Myelodysplastic syndromes are groups of clonal hematopoietic disorders characterized by refractory cytopenias with limited treatment options.1 These disorders are common in the elderly and impose a significant burden on health care resources. A stumbling block in the discovery of treatments of this disease has been the heterogeneity observed in subsets of this disease and the lack of a unifying pathophysiologic target. Our work has identified a kinase, p38 MAPK, that is activated in bone marrow cells of a large number of patients with MDS, even in those with different chromosomal alterations. Inhibition of this pathway leads to in vitro stimulation of hematopoiesis, suggesting that p38 MAPK pathway is a functionally important inhibitory pathway in MDS. p38 MAPK is not found to be activated in normal bone marrows, and consequently p38 inhibition does not have significant stimulatory effects on hematopoietic progenitors derived from normal bone marrows.
p38 MAPK was originally discovered as a stress-signaling kinase, and work has implicated it as an important mediator of apoptosis in neuronal, cardiac, immune, and other cells.41-44 Studies have shown that activation of the p38 MAPK pathway can oppose the proliferative effects of the Ras-Erk MAPK pathway and can lead to growth arrest and dormancy in tumors.45 Consistent with its cytostatic properties in nonhematopoietic cells, our work has shown an important role for p38 in cytokine-mediated inhibition of human hematopoiesis.29,30 In fact, a recent report implicates p38 in hematopoietic stem death induced by reactive oxygen species and suggests that p38 inhibition may be beneficial in prolonging stem-cell survival in ATM knockout mice.46 This report validates our findings of the important role of this MAP kinase in hematopoiesis, and it appears p38 activation is a downstream effector of many different cell-death pathways in hematopoietic stem cells.
Pharmacologic p38 inhibitors stimulate hematopoiesis in MDS CD34+ progenitors. MDS bone marrow–derived CD34+ cells from 19 patients were plated in methylcellulose in the presence and absence of p38 inhibitors SB203580 (5 μM and 10 μM), SD-282, and SCIO-469; inactive structural analog SB202474 (10 μM); and Mek-1 inhibitor PD98059 (10 μM). Colonies were scored at day 14, and results were expressed as means ± SEMs of 19 independent experiments.
Pharmacologic p38 inhibitors stimulate hematopoiesis in MDS CD34+ progenitors. MDS bone marrow–derived CD34+ cells from 19 patients were plated in methylcellulose in the presence and absence of p38 inhibitors SB203580 (5 μM and 10 μM), SD-282, and SCIO-469; inactive structural analog SB202474 (10 μM); and Mek-1 inhibitor PD98059 (10 μM). Colonies were scored at day 14, and results were expressed as means ± SEMs of 19 independent experiments.
Our data establish a correlation between marrow p38 activation and apoptosis that is characteristic of early-stage MDS. The lack of activation of p38 in nutritional deficiency anemias, chemotherapy-induced anemias, and other non-MDS cytopenias points to the relative specificity of p38 activation in low-risk/Int-1 MDS. At this stage of the disease, both normal and cytogenetically abnormal hematopoietic clones are found to exist in the marrow.47 It has been shown that abnormal MDS progenitor clones are resistant to apoptosis and have higher levels of antiapoptotic proteins Bcl-2 and Bax.6 Thus, it is possible that p38 inhibition may prevent cell death in the susceptible normal progenitors, thereby rescuing normal hematopoiesis in the early or low-grade stage of this disease. Because most MDS cases are low risk, and the morbidity experienced is due to low blood counts, hematopoietic recovery is a major therapeutic goal in treating these patients. The high numbers of apoptotic progenitors seen by us are consistent with similar high percentages seen in other reports in low-grade MDS.5 With disease progression toward high-risk stages, normal progenitors gradually undergo apoptosis, resulting in a bone marrow comprised mainly of the resistant abnormal clones. Thus, examination of the marrow at late stages reveals a low apoptotic index with higher percentages of myeloblasts. The low level of p38 activation correlates with the reduced apoptosis seen at this stage of the disease. Instead, constitutive activation of the antiapoptotic NFκB particularly in MDS progenitors was observed in a majority of high-risk MDS cases.48
MDS is highlighted by a stromal pathology of still unknown causes, which contributes to the pervasive presence of proinflammatory cytokines in the bone marrow. Dysregulation of various cytokines has been implicated in the pathogenesis of MDS.9,11,15-17 TNFα, TGFβ, and IFN-γ and -α are myelosuppressive cytokines that have been found to be elevated in serum as well as in the bone marrow of patients with MDS. Our earlier work has shown that p38 MAPK is activated by all of these cytokines in primary hematopoietic progenitors. We have also shown that p38 MAPK functions at a critical signaling juncture that links upstream signaling pathways induced by different immunosuppressive cytokines to a common effector pathway that leads to the inhibition of normal hematopoietic progenitor growth.4,6 Various strategies have targeted inhibitory cytokines in MDS with varying degrees of success. Clinical trials with monoclonal antibodies against TNF (infliximab)19 and soluble TNF receptors (etanercept)18 have yielded hematologic responses in small series of patients. The immunomodulatory (IMID) compounds, thalidomide,49 and the newer more efficacious lenalodimide50 also target TNFα and have been shown to reduce TNF mRNA51 and alter the cytokine milieu. Lenalodimide has been recently shown to be particularly efficacious of subsets of low-grade MDS with 5q deletion.50 Small molecule inhibitors of the VEGF receptor are presently in clinical trials for MDS. Even though these approaches are encouraging, they have not been useful for a majority of patients with this disease. Because p38 MAPK pathway is used by many cytokines, it is possible that targeting this pathway may have wider clinical benefit than treatments targeting individual cytokines alone (anti-TNF18 and anti-VEGF strategies) in MDS. We observed that the efficiency of p38 inhibitors in reversing TNF-mediated inhibition was slightly greater in the erythroid lineage in normal CD34 progenitors. This may be due to the different degrees of involvement of p38 signaling in distinct hematopoietic growth and differentiation pathways. Nevertheless, our results show that p38 inhibition affects both myeloid and erythroid progenitor growth, suggesting the role of this pathway in MDS disease subtypes involving different hematopoietic lineages.
SCIO-469 and SD-282 are novel small molecule inhibitors that occupy the ATP-binding site on the p38 kinase.37-39 Both of these are highly selective for p38α. Even though both the α and β isoforms are present in hematopoietic cells,52 our results with these inhibitors and siRNA suggest that p38α is the dominant p38 isoform involved in hematopoietic suppression in MDS. These results are consistent with our results previously observed with different classes of p38 inhibitors in a small series of cases.35 The efficacy of SCIO-469 in inhibiting cytokine signaling and production have provided preclinical rationale for its use in other cytokine-modulated diseases. It is presently being used in phase 2 clinical trials in rheumatoid arthritis and multiple myeloma. Our data support the efforts to bring this agent in clinical trials for patients with MDS.
Authorship
Contribution: T.A.N., M.M., P.P., and L.Z. designed the experiments and performed the research; J.Y.M., E.H., A.N.N., I.K., and L.S.H. performed the research and contributed the p38 inhibitors; M.E., L.S., and A.A.L. performed the research and contributed thepatient samples; L.C.P. contributed to the experimental design; Y.X., R.C., and S.P. contributed the patient samples; A.V. designed and performed the experiments and wrote the manuscript.
Conflict-of-interest disclosure: several of the authors (T.A.N., J.Y.M., E.H., I.K., A.N.N., and L.S.H.) are employed by a company or a competitor of a company (SCIOS, Inc) whose potential product was studied in the present work. The remaining authors declare no competing financial interests.
T.A.N., M.M., and M.E. contributed equally to this study.
Prepublished online as Blood First Edition Paper, August 29, 2006; DOI 10.1182/blood-2006-05-023093.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by the National Institutes of Health (1R01HL082946-01), by a grant from the Harris Methodist Foundation, by an VISN-17 award, and by the J.P. McCarthy Grant from the Community Foundation for Southwestern Michigan (A.V.).



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal