Comment on Pula et al, page 4035
In this issue of Blood, Pula and colleagues provide a novel mechanism for the negative regulatory role of platelet protein kinase C δ (PKCδ) that is independent of inside-out signaling, granular secretion, or early steps of GPVI signaling.
The initial step of agonist-induced platelet activation is platelet shape change, which is associated with intracellular calcium rise, phosphorylation of pleckstrin by PKC, and myosin light chain (MLC) by MLC kinase, followed by cytoskeletal rearrangement. Human platelets predominantly express 4 of the 12 known PKC isoforms.1 Although PKCα, β, and θ have been shown to positively regulate platelet activation, PKCδ is unique in that it plays a positive as well as a negative regulatory role.2,3
In this issue, Pula and colleagues provide convincing evidence regarding the negative role of PKCδ in platelet aggregation and reveal a novel mechanism for regulation of actin and filopodia. Filopodia are membranous protrusions formed and supported by bundles of actin filaments and are followed by lamellipodia formation leading to platelet spreading.4 Vasodilator-stimulated phosphoprotein (VASP) regulates actin polymerization and hence filopodia formation primarily through its anticapping activity. VASP is a major substrate of protein kinase A, protein kinase G, and PKC, which phosphorylate it on Ser157, Ser239, and Thr278. Phosphorylation of VASP on Ser157 is required for its anticapping activity.5
In previous studies using pharmacological agents, it was suggested that PKCδ negatively regulates collagen-induced dense granule secretion.3 Using PKCδ knockout mice, Pula and colleagues show that negative regulation of platelet aggregation by PKCδ is independent of inside-out signaling, dense granule secretion, and early GPVI signaling. They also provide evidence that PKCδ physically interacts with VASP and inhibits VASP phosphorylation on Ser157 by classical PKC (cPKC) isoforms, thus suppressing actin polymerization and filopodia formation. The fact that cPKCs' ability to phosphorylate other platelet proteins is unaffected by the inhibition or absence of PKCδ suggests that PKCδ does not directly inhibit the activity of these enzymes. This workundoubtedly provides provocative new ideas and will inspire new studies investigating how association of VASP and PKCδ inhibit phosphorylation of VASP on Ser157. The evidence presented strongly suggests that the PKCδ activity is necessary for the observed negative regulation.FIG1
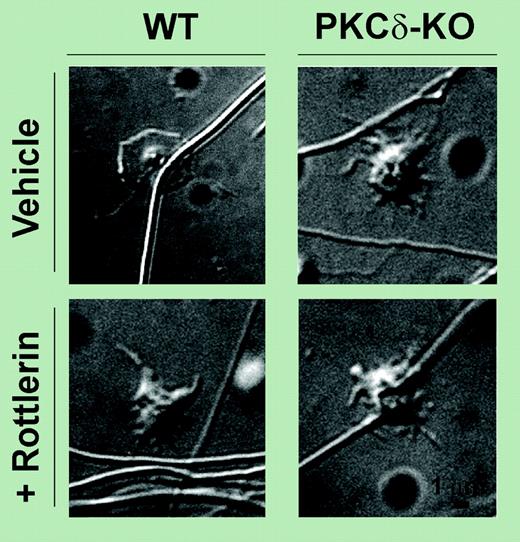
PKCδ negatively regulates filopodia formation in human platelets. See the complete figure in the article beginning on page 4035.
PKCδ negatively regulates filopodia formation in human platelets. See the complete figure in the article beginning on page 4035.
Future studies aimed at (1) the stoichiometry of PKCδ interaction with VASP, (2) how PKCδ activity is involved in inhibiting filopodia formation, (3) whether PKCδ activation is required for its interaction with VASP, (4) whether steric hindrance or conformational change resulting from PKCδ binding is responsible for decreased VASP phosphorylation on Ser157, and (5) whether PKCδ is constitutively associated with VASP in resting platelets or whether it becomes associated upon stimulation of platelets will be very interesting. Thus, this work, using a genetic approach, provides an intriguing avenue for further studies that might aid in design and development of novel therapeutic agents for the treatment of thrombotic disorders.
The author declares no competing financial interest. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal