Abstract
Regulatory T (Treg) cells accumulate in the lymphoid tissues of human immunodeficiency virus (HIV)–infected individuals, contributing to the inability of the immune system to control virus replication. We investigate here Treg-cell numbers and functional markers (FOXP3, CTLA-4, IDO, and TGF-β1) in lymphoid tissues from untreated infected hosts with progressive or nonprogressive disease (HIV-infected humans and simian immunodeficiency virus [SIV]–infected macaques). We found that increased numbers of FOXP3+ T cells as well as increased expression of Treg-cell–associated functional markers were detected only during progressive disease. Such increases were not correlated with immune activation. Of importance, a high-perforin/FOXP3 ratio was associated with nonprogressive disease, suggesting that the immune control of virus replication represents a balance between cell-mediated immune responses and Treg-cell–mediated counter regulation of such responses. Furthermore, using an in vitro model of Treg-cell–HIV interactions, we showed that exposure of Treg cells to HIV selectively promoted their survival via a CD4-gp120–dependent pathway, thus providing an underlying mechanism for the accumulation of Treg cells in infected hosts with active viral replication. Considered together, our findings imply that therapeutic manipulation of Treg-cell number and/or function could improve immune control of HIV infection.
Introduction
Defects in cell-mediated immunity (CMI) are important in chronic human immunodeficiency virus (HIV) infection.1 Failure of the immune system to clear infected cells results in high viral load and disease progression.2 Hallmarks of HIV-associated defects in CMI include impaired or absent CD4+ T-cell responses; inefficient CD8+ T-cell activity; and dysregulation of antigen-presenting cells (APCs). The majority of simian immunodeficiency virus (SIV)–infected macaques display similar features, although immunodeficiency generally develops more rapidly than in HIV-infected humans.3,4
However, viral replication is controlled in a minority of HIV-infected humans and SIV-infected macaques, resulting in a nonprogressive phenotype.5-8 Many nonprogressors maintain vigorous and broadly directed HIV-specific cytotoxic T-lymphocyte (CTL) and CD8-mediated HIV-suppressive activity.5-7,9-12 These findings imply a central role for CMI in the immunologic control of HIV and SIV infection. Nonetheless, the molecular bases for the progressive impairment of CMI in most infected hosts remain largely unknown.
Regulatory T (Treg) cells, a specialized subset of CD4+ T cells, were reported to suppress effector T-cell responses in chronic infections, including retroviral infections.13,14 Treg cells can suppress the function of APCs and of CD4+ and CD8+ effector T cells.15 Treg cells were proposed to suppress protective anti-HIV CMI based on the finding that in vitro depletion of Treg cells from circulating leukocytes of HIV-infected subjects resulted in increased anti-HIV T-cell responses.16-19 We and others have shown that Treg cells accumulate in the lymphoid tissue (LT) of HIV-infected individuals with active viral replication,20 and of acutely SIV-infected macaques, their number correlating inversely with the incidence of SIV-specific CD8+ T cells.21 However, the mechanisms underlying such accumulation, and its consequences on immune control of HIV/SIV infection, are unknown. Herein, we show that direct interactions between Treg cells and HIV promote Treg-cell survival, which in turn may lead to their accumulation in LTs of chronically infected hosts, where they are involved in reduced CMI activity and consequently in disease progression.
Patients, materials, and methods
Human subjects
Lymphoid tonsillar biopsies were obtained from 6 treatment-naive adults with chronic, progressing HIV-1 infection (HIVprog) and from 5 untreated adults with nonprogressing infection (HIVnp), all recruited at the Karolinska Institute, Stockholm, Sweden. The criteria for inclusion in the HIVnp group were (1) treatment naive; and (2) documented minimum of 5 years of infection with low viral load and stable CD4 counts. Those criteria predict a nonprogressing or slow-progressing disease.22 Lymphoid tonsillar biopsies were also obtained from 5 uninfected adults undergoing routine tonsillectomy due to mild sleep apnea syndrome (Karolinska Institute). Demographic and clinical data are presented in Table 1 and Table 2. All biopsies from HIVprogs, but none from HIVnps, were graded as follicular hyperplasia, which is prognostic of disease progression.23 The biopsies were snap-frozen and cryopreserved in OCT (Sakura, Torrance, CA). In addition, leukapheresis products were obtained from uninfected donors (Department of Blood Transfusion, NIH, Bethesda, MD). Informed consent in accordance with the Declaration of Helsinki was obtained from all subjects, and protocols were approved by the institutional review boards of the participating institutions (Karolinska Institute; NIH; Cincinnati Children's Hospital Medical Center, OH).
Clinical characteristics of included subjects
Subjects . | Sex . | Age, y . | CD4, cells/mm3 . | VL, copies/mL . | Treatment . | Stage* . | Duration, y† . |
|---|---|---|---|---|---|---|---|
| HIVprog 1‡ | M | 35 | 470 | 1 800 | None | A1 | >2 |
| HIVprog 2 | M | 42 | 464 | 241 861 | None | A1 | > 2 |
| HIVprog 3 | M | 23 | 282 | > 100 000 | None | A1 | > 2 |
| HIVprog 4 | F | 42 | 303 | 107 204 | None | A1 | > 2 |
| HIVprog 5 | M | 37 | 136 | 405 700 | None | C | > 2 |
| HIVprog 6 | M | 47 | 391 | 78 589 | None | A2 | > 2 |
| HIVnp 1 | F | 41 | 410 | 4 300 | None | A1 | 14 |
| HIVnp 2 | M | 36 | 510 | 300 | None | A1 | 15 |
| HIVnp 3 | M | 43 | 760 | < 50 | None | A1 | 15 |
| HIVnp 4 | F | 27 | 920 | < 50 | None | A1 | > 5 |
| HIVnp 5 | F | 34 | 620 | 200 | None | A1 | > 5 |
| Uninfected 1 | M | 37 | — | — | — | — | — |
| Uninfected 2 | F | 42 | — | — | — | — | — |
| Uninfected 3 | M | 39 | — | — | — | — | — |
| Uninfected 4 | M | 34 | — | — | — | — | — |
| Uninfected 5 | M | 28 | — | — | — | — | — |
Subjects . | Sex . | Age, y . | CD4, cells/mm3 . | VL, copies/mL . | Treatment . | Stage* . | Duration, y† . |
|---|---|---|---|---|---|---|---|
| HIVprog 1‡ | M | 35 | 470 | 1 800 | None | A1 | >2 |
| HIVprog 2 | M | 42 | 464 | 241 861 | None | A1 | > 2 |
| HIVprog 3 | M | 23 | 282 | > 100 000 | None | A1 | > 2 |
| HIVprog 4 | F | 42 | 303 | 107 204 | None | A1 | > 2 |
| HIVprog 5 | M | 37 | 136 | 405 700 | None | C | > 2 |
| HIVprog 6 | M | 47 | 391 | 78 589 | None | A2 | > 2 |
| HIVnp 1 | F | 41 | 410 | 4 300 | None | A1 | 14 |
| HIVnp 2 | M | 36 | 510 | 300 | None | A1 | 15 |
| HIVnp 3 | M | 43 | 760 | < 50 | None | A1 | 15 |
| HIVnp 4 | F | 27 | 920 | < 50 | None | A1 | > 5 |
| HIVnp 5 | F | 34 | 620 | 200 | None | A1 | > 5 |
| Uninfected 1 | M | 37 | — | — | — | — | — |
| Uninfected 2 | F | 42 | — | — | — | — | — |
| Uninfected 3 | M | 39 | — | — | — | — | — |
| Uninfected 4 | M | 34 | — | — | — | — | — |
| Uninfected 5 | M | 28 | — | — | — | — | — |
VL indicates viral load; —, not applicable.
Disease stage, according to CDC classification. In addition, all biopsies from the HIVprog, but none from the HIVnp, were graded as follicular hyperplasia, which is prognostic of disease progression.23
Documented duration of HIV-1 infection, defined as years of known seropositivity.
Although his CD4 counts was relatively high and his VL was low at the time of biopsy, HIVprog 1 did not meet the definition of the nonprogressors (ie, stable CD4 counts and low VL for a minimum of 5 years). Moreover, subsequent follow-up of HIVprog 1 has shown a pattern toward progression, with a steady VL increase (7 600 and 22 000 copies/mL at 3 and 5.5 years after biopsy, respectively).
Extended clinical follow-up of HIVnp
. | Before biopsy (y) . | . | Biopsy . | . | After biopsy (y) . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Subjects . | CD4, cells/mm3 . | VL, copies/mL . | CD4, cells/mm3 . | VL, copies/mL . | CD4, cells/mm3 . | VL, copies/mL . | |||
| HIVnp 1 | 770 (2)* | 500 (2) | 410 | 4300 | 510 (1)* | 1400 (1) | |||
| HIVnp 2 | 880 (3) | 499 (3) | 510 | 300 | 772 (3) | 6300 (3) | |||
| HIVnp 3 | 1050 (1) | < 50 (1) | 760 | < 50 | 1179 (4) | < 50 (4) | |||
| HIVnp 4 | 600 (2) | 200 (2) | 920 | < 50 | N/A† | N/A† | |||
| HIVnp 5 | 820 (2) | < 50 | 620 | 200 | 739 (5) | 200 (5) | |||
. | Before biopsy (y) . | . | Biopsy . | . | After biopsy (y) . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Subjects . | CD4, cells/mm3 . | VL, copies/mL . | CD4, cells/mm3 . | VL, copies/mL . | CD4, cells/mm3 . | VL, copies/mL . | |||
| HIVnp 1 | 770 (2)* | 500 (2) | 410 | 4300 | 510 (1)* | 1400 (1) | |||
| HIVnp 2 | 880 (3) | 499 (3) | 510 | 300 | 772 (3) | 6300 (3) | |||
| HIVnp 3 | 1050 (1) | < 50 (1) | 760 | < 50 | 1179 (4) | < 50 (4) | |||
| HIVnp 4 | 600 (2) | 200 (2) | 920 | < 50 | N/A† | N/A† | |||
| HIVnp 5 | 820 (2) | < 50 | 620 | 200 | 739 (5) | 200 (5) | |||
Number of years before or after biopsy when data were collected.
N/A indicates not available. This individual was put on highly active antiretroviral therapy (HAART) shortly after the biopsy was obtained, because she became pregnant. She did not have further follow-up.
Macaques
Sixteen colony-bred Indian rhesus macaques (Macaca mulatta) were obtained from Covance Research Products (Alice, TX) and handled in accordance with the standards of the American Association for the Accreditation of Laboratory Animal Care. Fourteen of these macaques were infected with SIVmac251 for 2 years prior to lymph node excision (Table 3). SIVmac251 RNA was quantified in plasma by nucleic acid sequence–based amplification (detection limit: 2 × 103 RNA copies/mL). Two animals were uninfected. The protocol was approved by the NIH animal review board. Whole axillary lymph nodes were mechanically disrupted and mononuclear cells were isolated by density centrifugation (Ficoll-Hypaque; Amersham, Piscataway, NJ).
Viral loads at death
Animal no. . | VL, copies/mL . |
|---|---|
| Uninfected | |
| 280 | N/A |
| 304 | N/A |
| SIVnp | |
| 883 | < 2 000 |
| 884 | < 2 000 |
| 207 | < 2 000 |
| 916 | < 2 000 |
| 299 | < 2 000 |
| 878 | 2 092 |
| 879 | < 2 000 |
| SIVprog | |
| 911 | 4 650 525 |
| 876 | 2 435 |
| 890 | 28 016 |
| 202 | 14 467 |
| 547 | 40 075* |
| 173 | 380 647* |
| 909 | 3 603 |
Animal no. . | VL, copies/mL . |
|---|---|
| Uninfected | |
| 280 | N/A |
| 304 | N/A |
| SIVnp | |
| 883 | < 2 000 |
| 884 | < 2 000 |
| 207 | < 2 000 |
| 916 | < 2 000 |
| 299 | < 2 000 |
| 878 | 2 092 |
| 879 | < 2 000 |
| SIVprog | |
| 911 | 4 650 525 |
| 876 | 2 435 |
| 890 | 28 016 |
| 202 | 14 467 |
| 547 | 40 075* |
| 173 | 380 647* |
| 909 | 3 603 |
VL measured 12 weeks before death.
Determination of mRNA expression in macaque LT
Total RNA was extracted from macaque LT mononuclear cells using the guanidium thiocyanate–phenol–chloroform method, modified for TRIzol (Invitrogen, Carlsbad, CA). RNA was reverse-transcribed using random hexanucleotide primers and oligo dT, and Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). Quantification of FOXP3, IFN-γ, granzyme B, perforin, CTLA-4, IDO, TGF-β1, IL-10, CD4, and GAPDH mRNA was performed by real-time polymerase chain reaction (PCR) (ABI Prism 7900HT; Applied Biosystems, Foster City, CA) using a SYBR green PCR mix (Qiagen, Valencia, CA) and specific primers designed from the macaque gene sequences. Results are presented as relative units (RU) obtained by normalizing the target gene mRNA with either the corresponding CD4 or GAPDH mRNA values.
Determination of mRNA expression in human cells and tissues
Total RNA was extracted from cells and human LT using RNAeasy mini kits, following the manufacturer's instruction (Qiagen), as described.20 RNA was reverse-transcribed using superscript reverse transcriptase (Invitrogen) and random hexanucleotide primers. Reverse-transcription (RT) product was amplified using real-time PCR, performed in a Light-Cycler (Roche, Indianapolis, IN) using a SYBR green PCR kit (Roche) and specific primers for the analyzed human genes.24
Flow cytometry analysis of Treg-cell markers
Cells were stained with anti–CD25-APC (M-A251; BD PharMingen, San Diego, CA) or anti–GITR-PE (110416; R&D Systems, Minneapolis, MN) after incubation with human IgG (10 μg/mL; Sigma, St Louis, MO) to block Fc receptors. Cells were then stained with anti–FOXP3-FITC (PCH101; eBiosciences, San Diego, CA), according to eBiosciences's instructions. In another tube, anti–GITR-PE was omitted and anti–CTLA4-PE (14D3; eBiosciences) was added at the same time as anti-FOXP3 Ab. The appropriate isotype-matched control Abs were used to define positivity. Marker expression was analyzed using a FACSCalibur and CellQuest software (BD PharMingen). A minimum of 10 000 cells/tube was analyzed.
Immunohistochemistry and confocal analysis of human LT
Biopsy samples (8 μm thick) were fixed in 2% formaldehyde, and blocked for endogenous biotin (Vector Laboratories, Burlingame, CA). Anti–human CTLA-4, CD4, CD8 (BD PharMingen), CD25 (Pelicluster; Sanquin, Amsterdam, The Netherlands), CD69 (DakoCytomation, Glostrup, Denmark), FOXP3 (Ab2481; Novus Biologicals, Littleton, CO), perforin, and granzyme B (Endogen, Rockford, IL), and irrelevant isotype matched Ab were used.20 The mean size of the scanned area per section was 2.1 × 106 μm2. Doubleand single-positive stained cells were quantified in 15 high-power fields using Qwin 550 software (Leica Imaging Systems, Cambridge, United Kingdom) and a confocal microscope (Leica TCS SP2 AOBS; Leica, Heidelberg, Germany). A blinded investigator carried out image analyses.
In vitro exposure to inactivated HIV
CD4+ T cells were purified from the blood of HIV-uninfected donors by negative selection (CD4 T-cell isolation KitII human; Miltenyi, Auburn, CA). Cells were cultured in presence of aldrithiol-2–inactivated HIV-125,26 (AT-2 HIV), kindly provided by Dr J. Lifson (AIDS Vaccine Program; SAIC Frederick, Frederick, MD). AT-2 HIV was used at the concentration of 1 μg HIV p24gag equivalent/mL, which corresponds to a gp120 concentration of approximately 1 nM.27 Microvesicles, isolated from uninfected cultures of CEM X 174 cells following the same procedures as those used to prepare AT-2 HIV, were used as negative control. These vesicles control for the effect of AT-2 treatment, as well as the effect of cellular proteins budding from vesicles.28 Microvesicles were added at a concentration that provided an equal amount of total protein as AT-2 HIV. In most experiments, HIVMN was used. In some experiments, HIVADA was used at the same concentration as HIVMN.
Total CD4+ T cells (2 × 106) were cultured in complete RPMI medium supplemented with 2% human AB+ serum in presence of AT-2 HIV or microvesicles. At day 1, IL-2 (20 UI/mL; AIDS Research and Reference Reagent Program, NIH) was added to every well. Cells were prepared for mRNA extraction or fluorescence-activated cell sorter (FACS) analysis of Treg-cell markers at different times after culture initiation. In some experiments, CD4+CD25+ T cells were deleted from total CD4+ cells (CD25 purification kit; Miltenyi). Purity of more than 90% was routinely achieved, as assessed by FACS analysis. Cells were exposed to AT-2 HIV as described for total CD4+ T cells.
In some experiments, soluble CD4-IgG (sCD4, which encompasses the CD4 N-terminal 183 amino-acid residues,29 10 μg/mL; AIDS Research and Reference Reagent Program) was added to AT-2 HIV–exposed CD4+ T cells.
In some experiments, purified CD4+ T cells were exposed to anti-CD4 Ab, which recognizes the domain 1 of the CD4 molecule (clone QS4120), or to an isotype-matched control Ab (Ancell, Bayport, MN; both at 10 μg/mL) for 1 hour at +4°C, washed, and then cultured as described for AT-2 HIV–exposed CD4+ T cells.
Determination of Treg-cell apoptosis
CD4+CD25+ cells, purified by positive selection (purity of > 90%, CD25 purification kit; Miltenyi) were exposed to AT-2 HIV or microvesicles, as described for total CD4+ T cells. At day 5, cells were stained for surface expression of annexin V, following BD PharMingen's protocol.
In vitro Treg-cell-suppressive activity
Purified CD4+CD25+ cells were exposed to AT-2 HIV or microvesicles. At day 5, cells were washed 3 times to remove unbound virus or microvesicles, and live cells were counted and mixed at a ratio of 1:1 with autologous effector CD4+CD25– T cells (5 × 105/well). Effector cells had been labeled with 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE, 1.25 μM for 5 minutes, followed by several washes; Invitrogen). Since, in our experience, the PHA-induced proliferation of highly purified CD4+ T cells is not optimal but is enhanced by addition of APCs, autologous irradiated elutriated monocytes (5 × 104/well) were added to all cultures. Effector T cells were stimulated with PHA (PHA-M, 2 μg/mL; Sigma) for 3 days in presence or absence of Treg cells. Effector T cells were also cultured with APCs alone, as a negative control. CFSE levels were determined by FACS, acquiring a minimum of 20 000 labeled cells.
Statistical analysis
Gene expression in biopsies was statistically analyzed using Mann-Whitney U tests. Percentages of positive cells were assessed by ordinary leastsquares regression after logit transformation of the data. Correlations were determined with Spearman rank correlation. In vitro expression of Treg-cell markers was analyzed using paired t tests. A 2-tailed P value less than .05 was considered to be significant.
Results
Nonprogressors exhibit low FOXP3 expression in lymphoid tissues
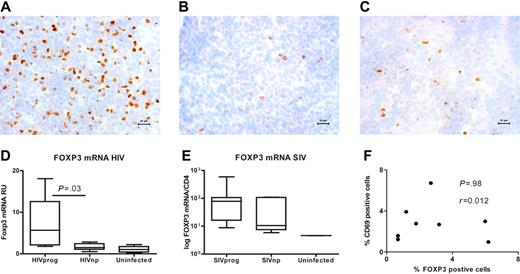
We and others have previously shown that active replicating HIV and SIV infection is associated with the accumulation of Treg cells in LTs, the major site of virus replication.20,21 To determine whether such accumulation has any effect on HIV disease progression, we compared FOXP3 protein expression at the single-cell level in the tonsils of 5 HIVnps and 6 HIVprogs (see Table 1 and Table 2 for description of clinical characteristics). Tissues from 5 HIV-uninfected adults were also analyzed. Strikingly, the number of FOXP3+ cells was 5-fold lower in HIVnps than in HIVprogs, but similar to uninfected subjects (Figure 1A-C). Similar significant differences were observed when FOXP3 mRNA levels were quantified in the same tissues (Figure 1D). Of importance, FOXP3 expression in HIVprogs was not directly associated with immune activation, since the percentage of FOXP3+ and CD69+ cells was not correlated in HIV-infected subjects (P = .98) (Figure 1F).
To validate our findings from HIV-infected individuals in the macaque model, we tested axillary lymph nodes (LNs) from 14 SIV-infected macaques (Table 3). Seven of these macaques were nonprogressors and had persistently undetectable viral loads (SIVnps), whereas the remaining 7 exhibited progressive infection and persistent viremia (SIVprogs). LNs were also collected in 2 uninfected macaques. A trend toward lower FOXP3 mRNA expression (approximately 3-fold) was also observed in the LTs from SIVnps, compared with SIVprogs (Figure 1E). These findings suggest that extensive Treg-cell accumulation within LTs is a feature of chronic, progressive HIV or SIV infection, but not of nonprogressive infection.
Low expression of FOXP3 in LT from HIVnp and SIVnp. (A-C) Photomicrograph of FOXP3 protein–expressing cells (brown) in LT from a representative HIVprog (n = 6) (A), HIVnp (n = 5) (B), and uninfected individual (n = 5) (C). Cell nuclei were counterstained in blue; the micron bar in bottom right corner indicates 20 μm. Images were acquired using a Leica DMR-X microscope coupled to a Leica DC500 digital camera (Leica, Wetzlar, Germany), using the image analysis system Quantimet Q550 (Leica Imaging Systems, Cambridge, United Kingdom). The image was acquired using a 40×/0.75 numerical aperture (NA) oil objective. The staining reactions were developed using diaminobenzidine tetrahydrochloride and hematoxylin. (D) FOXP3 mRNA expression was significantly decreased in LT of HIVnp. Results are expressed in RU after normalization on CD4 mRNA. Similar results were obtained when FOXP3 was normalized on GAPDH expression (P = .004, not shown). Horizontal bars within boxes correspond to the median; box limits correspond to the 25th and 75th percentiles; vertical lines indicate range. (E) Median FOXP3 mRNA expression was decreased in LT from 7 SIVnps, compared with that in 7 SIVprogs. Results are expressed in RU after normalization on CD4 mRNA. (F) Percentages of CD69+ and FoxP3+ cells were not correlated in LT of HIV-infected individuals (r = 0.012, P = .98; Spearman correlation).
Low expression of FOXP3 in LT from HIVnp and SIVnp. (A-C) Photomicrograph of FOXP3 protein–expressing cells (brown) in LT from a representative HIVprog (n = 6) (A), HIVnp (n = 5) (B), and uninfected individual (n = 5) (C). Cell nuclei were counterstained in blue; the micron bar in bottom right corner indicates 20 μm. Images were acquired using a Leica DMR-X microscope coupled to a Leica DC500 digital camera (Leica, Wetzlar, Germany), using the image analysis system Quantimet Q550 (Leica Imaging Systems, Cambridge, United Kingdom). The image was acquired using a 40×/0.75 numerical aperture (NA) oil objective. The staining reactions were developed using diaminobenzidine tetrahydrochloride and hematoxylin. (D) FOXP3 mRNA expression was significantly decreased in LT of HIVnp. Results are expressed in RU after normalization on CD4 mRNA. Similar results were obtained when FOXP3 was normalized on GAPDH expression (P = .004, not shown). Horizontal bars within boxes correspond to the median; box limits correspond to the 25th and 75th percentiles; vertical lines indicate range. (E) Median FOXP3 mRNA expression was decreased in LT from 7 SIVnps, compared with that in 7 SIVprogs. Results are expressed in RU after normalization on CD4 mRNA. (F) Percentages of CD69+ and FoxP3+ cells were not correlated in LT of HIV-infected individuals (r = 0.012, P = .98; Spearman correlation).
Nonprogressors exhibit low expression of markers of Treg-cell function in lymphoid tissues
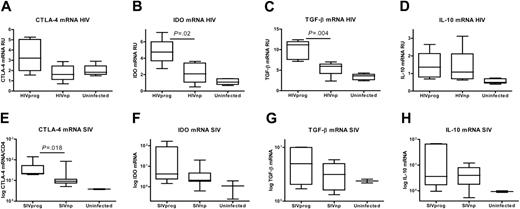
We tested whether the low FOXP3 expression in HIVnps and SIVnps was accompanied by low expression of molecules associated with Treg-cell function, such as CTLA-4. HIVprogs showed a trend to higher CTLA-4 than HIVnps (approximately 2-fold, P = .09) and uninfected controls (Figure 2A). Similar results were obtained after analysis of CTLA-4 protein expression, which was 6-fold higher in HIVprogs than in HIVnps (data not shown). The majority (> 85%) of FOXP3+ cells in the LTs of the different groups coexpressed CTLA-4 (data not shown). Because CTLA-4–expressing Treg cells were shown to induce the tryptophancatabolizing enzyme indoleamine 2-dioxygenase (IDO) by APCs,30 we analyzed IDO mRNA in LT. IDO levels in HIVnps were lower than in HIVprogs (P = .02), but comparable with uninfected controls (Figure 2B). These results were confirmed in SIV-infected macaques: SIVnps expressed lower levels of CTLA-4 (P = .02) than SIVprogs and displayed a trend toward decreased IDO (8-fold decrease; P = .2) mRNA (Figure 2E-F).
Low expression of mediators associated with Treg-cell function in LT of HIVnp and SIVnp. mRNA levels in tissues from human individuals (A-D) and macaques (E-H) are expressed in RU after normalization on GAPDH mRNA.
Low expression of mediators associated with Treg-cell function in LT of HIVnp and SIVnp. mRNA levels in tissues from human individuals (A-D) and macaques (E-H) are expressed in RU after normalization on GAPDH mRNA.
Suppressive cytokines, particularly TGF-β1 and IL-10, are also implicated in Treg-cell-mediated suppression. In human LTs, TGF-β1 mRNA expression was lower in HIVnps than in HIVprogs (P = .004; Figure 2C) and comparable with uninfected controls. SIVnps also exhibited lower TGF-β1 expression compared with SIVprogs (Figure 2G). In contrast, expression of IL-10 mRNA was equally up-regulated in both HIVand SIV-infected hosts, compared with uninfected hosts (Figure 2D,H). Therefore, low FOXP3 levels in LTs from nonprogressor subjects were associated with concomitant low levels of CTLA-4, IDO, and TGF-β1, but not of IL-10.
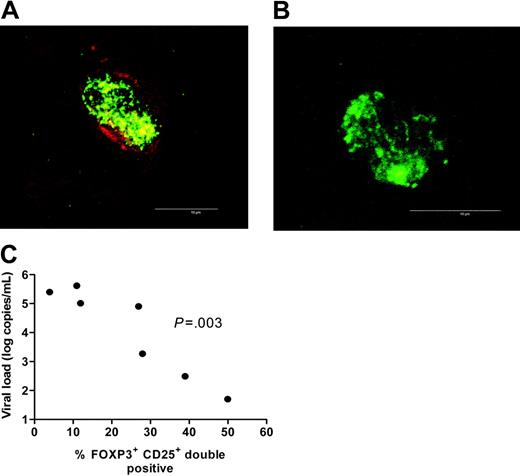
Plasma viral load is negatively correlated with expression of CD25 by FOXP3+ T cells. (A-B) High-magnification confocal micrograph of a FOXP3 (green) and CD25 (red) double-positive cell (A) and a FOXP3 (green) single-positive cell (B). Microbar in bottom right corner indicates 10 μm. Images were acquired using a Leica confocal scanner TCS SP II, coupled to a Leica DMR microscope (Leica, Wetzlar, Germany) using a 63×/0.75 NA oil objective. The staining reactions were developed using streptavidin-conjugated fluorophores (Alexa Fluor 488 and 594; Molecular Probes, Eugene, OR). (C) Plasma viral load was negatively correlated to the expression of CD25 by FOXP3+ T cells (r = –0.96, P = .003; Spearman correlation). This characterization was done in 7 HIV-infected patients across a wide span of viral loads (5 HIVprogs and 2 HIVnps were included in the analysis).
Plasma viral load is negatively correlated with expression of CD25 by FOXP3+ T cells. (A-B) High-magnification confocal micrograph of a FOXP3 (green) and CD25 (red) double-positive cell (A) and a FOXP3 (green) single-positive cell (B). Microbar in bottom right corner indicates 10 μm. Images were acquired using a Leica confocal scanner TCS SP II, coupled to a Leica DMR microscope (Leica, Wetzlar, Germany) using a 63×/0.75 NA oil objective. The staining reactions were developed using streptavidin-conjugated fluorophores (Alexa Fluor 488 and 594; Molecular Probes, Eugene, OR). (C) Plasma viral load was negatively correlated to the expression of CD25 by FOXP3+ T cells (r = –0.96, P = .003; Spearman correlation). This characterization was done in 7 HIV-infected patients across a wide span of viral loads (5 HIVprogs and 2 HIVnps were included in the analysis).
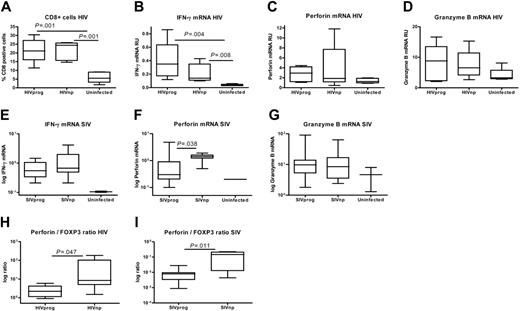
Treg-cell accumulation in progressor hosts interferes with CMI. (A) Significant increase of percentages of CD8+ T cells in LT of 6 HIVprogs and 5 HIVnps compared with 5 uninfected controls. (B-D) Expression of IFN-γ (B), perforin (C), and granzyme B (D) mRNA. Results are expressed in RU after normalization on GAPDH mRNA. Phenotyping of LT perforinand granzyme B–expressing cells confirmed that they were CD3+ and CD8+ (> 95%; data not shown). (E-G) Expression of IFN-γ (E), perforin (F), and granzyme B (G) mRNA in LT of SIVprogs, SIVnps, and uninfected animals. Results are expressed in RU after normalization on GAPDH mRNA. (H-I) Increased ratio of perforin to FOXP3 mRNA expression in nonprogressors compared with progressors (Mann-Whitney test).
Treg-cell accumulation in progressor hosts interferes with CMI. (A) Significant increase of percentages of CD8+ T cells in LT of 6 HIVprogs and 5 HIVnps compared with 5 uninfected controls. (B-D) Expression of IFN-γ (B), perforin (C), and granzyme B (D) mRNA. Results are expressed in RU after normalization on GAPDH mRNA. Phenotyping of LT perforinand granzyme B–expressing cells confirmed that they were CD3+ and CD8+ (> 95%; data not shown). (E-G) Expression of IFN-γ (E), perforin (F), and granzyme B (G) mRNA in LT of SIVprogs, SIVnps, and uninfected animals. Results are expressed in RU after normalization on GAPDH mRNA. (H-I) Increased ratio of perforin to FOXP3 mRNA expression in nonprogressors compared with progressors (Mann-Whitney test).
Plasma viral load inversely correlates with expression of CD25 by FOXP3+ T cells
CD25 has been widely used to define Treg cells in both human and murine systems. Therefore, we tested whether CD25 and FOXP3 were coexpressed in the different subject populations. Coexpression of the 2 markers varied considerably among the different groups, with medians of 19.4%, 44%, and 75% of FOXP3+ cells also being CD25+ in HIVprogs, HIVnps, and uninfected donors, respectively (Figure 3A-B). Of importance, the percentage of FOXP3+CD25+ cells inversely correlated with plasma viral load in HIV-infected subjects (Figure 3C). These results suggest that Treg cells that have expanded/accumulated in LTs during HIV infection down-regulate CD25 expression in vivo.
Treg-cell accumulation in progressor hosts interferes with CMI
To test whether accumulation of Treg cells in LT interferes with efficient CMI, we determined CD8+ T-cell numbers and expression of CMI effector molecules in the same LT biopsies. CD8 numbers in HIVnps were increased compared with uninfected controls but similar to HIVprogs (Figure 4A). We also quantified the mRNA expression of CMI effector cytokine IFN-γ, and the CTLassociated molecules perforin and granzyme B. LTs from both HIVnps and HIVprogs expressed more IFN-γ compared with uninfected controls (Figure 4B). In addition, we observed a trend toward increased granzyme B and perforin expression in both HIVnps and HIVprogs compared with uninfected controls (Figure 4C-D). The same parameters were assessed in macaques. IFN-γ and granzyme B mRNA expression was approximately 4to 5-fold higher in SIVnps and SIVprogs compared with uninfected macaques (Figure 4E,G). In contrast, significantly higher perforin mRNA levels were measured in LTs from SIVnps than SIVprogs (P = .04) (Figure 4F). Strikingly, a high perforin/FOXP3 ratio was associated with nonprogressive disease in both humans and macaques (Figure 4H-I). Collectively, these data suggest that control of virus replication depends on preferential activation of CMI in the absence of accumulating Treg cells within the LTs.
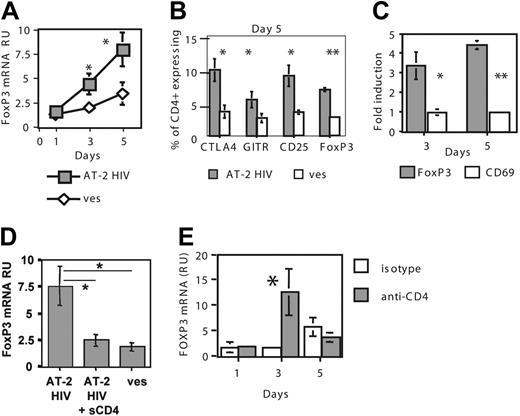
HIV directly induces accumulation of functional Treg cells through gp120-CD4 interactions. (A) Exposure of CD4+ T cells to AT-2 HIV increases FOXP3 mRNA expression. CD4+ T cells were cultured in presence of either AT-2 HIVMN or control microvesicles (ves). FOXP3 mRNA RU were calculated after normalization on CD4 mRNA levels. CD4 mRNA levels were not affected by AT-2 HIV exposure (results not shown). Results are expressed as the mean ± SE of data obtained in 7 donors. Asterisks indicate a significant difference by paired t test (*P < .05; **P < .005). (B) Increased expression of Treg-cell markers in AT-2 HIV–exposed T cells. Percentage of cells expressing each marker was determined by FACS. Results represent the mean ± SE of data obtained in 4 donors. Similar results were obtained at day 3 (not shown). (C) AT-2 HIV exposure does not induce CD69 up-regulation. Results represent the mean ± SE of data obtained in 4 donors. (D) sCD4 abrogates AT-2 HIV–mediated FOXP3 increased expression. Results represent the mean ± SE of data obtained in 5 donors at day 5. (E) CD4 engagement by anti-CD4 Ab induces increased FOXP3 mRNA expression. Results represent the mean ± SE of data obtained in 3 donors.
HIV directly induces accumulation of functional Treg cells through gp120-CD4 interactions. (A) Exposure of CD4+ T cells to AT-2 HIV increases FOXP3 mRNA expression. CD4+ T cells were cultured in presence of either AT-2 HIVMN or control microvesicles (ves). FOXP3 mRNA RU were calculated after normalization on CD4 mRNA levels. CD4 mRNA levels were not affected by AT-2 HIV exposure (results not shown). Results are expressed as the mean ± SE of data obtained in 7 donors. Asterisks indicate a significant difference by paired t test (*P < .05; **P < .005). (B) Increased expression of Treg-cell markers in AT-2 HIV–exposed T cells. Percentage of cells expressing each marker was determined by FACS. Results represent the mean ± SE of data obtained in 4 donors. Similar results were obtained at day 3 (not shown). (C) AT-2 HIV exposure does not induce CD69 up-regulation. Results represent the mean ± SE of data obtained in 4 donors. (D) sCD4 abrogates AT-2 HIV–mediated FOXP3 increased expression. Results represent the mean ± SE of data obtained in 5 donors at day 5. (E) CD4 engagement by anti-CD4 Ab induces increased FOXP3 mRNA expression. Results represent the mean ± SE of data obtained in 3 donors.
HIV induces Treg-cell accumulation through HIV gp120–CD4 interactions
To determine whether HIV plays a direct role in Treg-cell accumulation in LTs of infected hosts, we developed an in vitro model, in which CD4+ T cells from uninfected donors were exposed to inactivated HIV. We used inactivated virions because less than 1% of HIV virions are demonstrably infectious; therefore, noninfectious interactions between HIV and CD4 occur at high frequency in HIV-infected individuals.2,31 We used aldrithiol-2 (AT-2)–inactivated HIV-1, which is reverse-transcription deficient, but retains the structural and functional integrity of the envelope.25 Microvesicles prepared from AT-2–treated uninfected cells were used as control. FOXP3 mRNA levels were significantly increased in AT-2 HIV–exposed CD4+ T cells on day 3 and day 5 (Figure 5A). Increased percentages of cells expressing FOXP3 protein were detected at the same times (Figure 5B). Exposure to AT-2 HIV also increased expression of CTLA-4, GITR, and CD25 on day 3 (not shown) and day 5 (Figure 5B). Of interest, most of the HIV-exposed FOXP3+ cells also expressed CTLA-4 (75.6% ± 6.6%) and CD25 (71.1% ± 3.2%), but not GITR (43.8% ± 6.1%), on day 5, suggesting that these molecules may be differently regulated. Because all Treg-cell markers can be expressed in activated non-Treg cells, including transient expression of FOXP3,32 we determined the mRNA expression of the activation marker CD69 in AT-2 HIV– and microvesicle-exposed cells. CD69 expression was not increased on day 3 or day 5 (P = .39 and P = .67, respectively, Figure 5C), suggesting that increased expression of Treg-cell markers was not due to immune activation.
We then determined whether HIV gp120–CD4 interactions were involved in increased FOXP3 expression. Soluble CD4-IgG (sCD4) almost completely abrogated HIV-mediated increases in FOXP3 expression (Figure 5D). Accordingly, heat treatment, which promotes dissociation of gp120 from the virions,33 abolished the effect of AT-2 HIV on FOXP3 expression (data not shown). No difference was observed between the X4 MN and R5 ADA strains in their ability to induce FOXP3 at day 5 (fold-induction of 8.4 ± 2.3 and 7.1 ± 1.9 for MN and ADA, respectively, 3 subjects, P = .27), suggesting a major role for CD4-gp120 interactions. Finally, CD4+ T cells exposed to an anti-CD4 Ab that binds the same domain of the CD4 molecule as HIV gp120 exhibited higher FOXP3 mRNA expression at day 3 than isotype-exposed cells (Figure 5E), suggesting that CD4 engagement is sufficient to induce FOXP3 up-regulation.
HIV decreases apoptosis in CD25+ cells
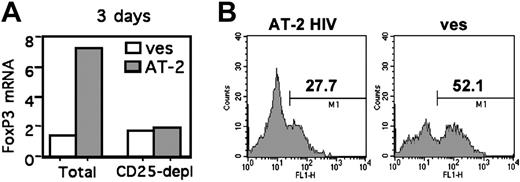
We investigated whether HIV-mediated increased FOXP3 was due to the induction of FOXP3 in non-Treg cells or the survival/expansion of pre-existing Treg cells. FOXP3 mRNA levels were increased in total CD4+ T cells but not in CD25– cells following AT-2 HIV exposure (Figure 6A), arguing against the induction of FOXP3 in non-Treg cells. We then determined whether increased FOXP3 expression was due to increased survival of HIV-exposed Treg cells, by analyzing the level of apoptosis in purified Treg cells exposed to AT-2 HIV or microvesicles. At day 5, the proportion of annexin V+ cells was approximately 2-fold lower in HIV-exposed Treg cells (27.5% ± 4.9%) compared with microvesicle-exposed Treg cells (58.9% ± 3.2%; P = .03, 3 donors, Figure 6B). These data strongly suggest that HIV exposure results in increased survival of pre-existing Treg cells, and not in induction of FOXP3 in non-Treg cells.
TGF-β1 has been described as a cytokine involved in Treg-cell survival/expansion.34 Therefore, we determined TGF-β1 mRNA levels in AT-2 HIV– and vesicle-exposed total CD4+ T-cell cultures. No increase in TGF-β1 expression was found in AT-2 HIV–exposed T cells at any of the studied time points (days 1, 3, and 5 after exposure; data not shown), suggesting that TGF-β1 induction does not account for enhanced survival of in vitro HIV-exposed Treg cells.
HIV decreases Treg-cell apoptosis. (A) Depletion of CD25+ T cells before HIV exposure abrogates FOXP3 induction. CD4+ T cells from 4 individuals were either left unseparated (total) or depleted of CD25+ cells (depl) by negative selection, and were cultured in presence of AT-2 HIV or microvesicles. FOXP3 mRNA expression was determined at day 5. Similar results were obtained at day 3 (not shown). (B) Exposure of CD4+CD25+ T cells to AT-2 HIV decreases the number of apoptotic cells. Purified CD4+CD25+ T cells were cultured in presence of AT-2 HIV, or microvesicles for 5 days. Percentages of annexin V+ cells (determined in comparison with unstained controls) in 1 donor, representative of 3 donors, are shown.
HIV decreases Treg-cell apoptosis. (A) Depletion of CD25+ T cells before HIV exposure abrogates FOXP3 induction. CD4+ T cells from 4 individuals were either left unseparated (total) or depleted of CD25+ cells (depl) by negative selection, and were cultured in presence of AT-2 HIV or microvesicles. FOXP3 mRNA expression was determined at day 5. Similar results were obtained at day 3 (not shown). (B) Exposure of CD4+CD25+ T cells to AT-2 HIV decreases the number of apoptotic cells. Purified CD4+CD25+ T cells were cultured in presence of AT-2 HIV, or microvesicles for 5 days. Percentages of annexin V+ cells (determined in comparison with unstained controls) in 1 donor, representative of 3 donors, are shown.
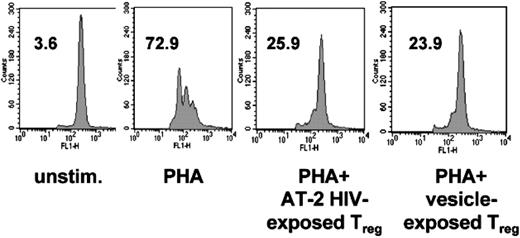
HIV-exposed Treg cells maintain strong suppressive activity. Purified CD4+CD25+ T cells were cultured in presence of AT-2 HIV, or microvesicles. At day 4, cells were mixed with CFSE-labeled autologous CD4+CD25– T cells, and stimulated with PHA for 3 days in presence of APCs. Effector T cells were also cultured with PHA and APCs (PHA), and with APCs alone (unstim). Numbers indicate the percentages of effector cells that had undergone at least one division cycle. Results from 1 donor, representative of 3, are shown.
HIV-exposed Treg cells maintain strong suppressive activity. Purified CD4+CD25+ T cells were cultured in presence of AT-2 HIV, or microvesicles. At day 4, cells were mixed with CFSE-labeled autologous CD4+CD25– T cells, and stimulated with PHA for 3 days in presence of APCs. Effector T cells were also cultured with PHA and APCs (PHA), and with APCs alone (unstim). Numbers indicate the percentages of effector cells that had undergone at least one division cycle. Results from 1 donor, representative of 3, are shown.
HIV-exposed Treg cells maintain strong suppressive activity
HIV-exposed Treg cells maintained strong suppressive activity, on a per-cell basis, as evidenced by their capacity to inhibit PHAstimulated effector cell division, significantly reducing the percentage of CFSE-low effector cells after PHA stimulation from 66.7% ± 2.5% to 35.6% ± 3.6% (P = .05, paired t test; Figure 7). AT-2 HIV– and microvesicle-exposed Treg cells were equally suppressive (paired t test; P = .8; Figure 7). These data suggest that HIV-exposed Treg cells remain functional and could hamper in vivo effector immune responses.
Discussion
A growing body of evidence demonstrates that Treg cells interfere with protective immune responses in diverse chronic infections. Our data uniquely demonstrate that immune-mediated control of viral replication is associated with low numbers of LT FOXP3+CD4+ T cells, whereas uncontrolled active viral replication is associated with high numbers of these cells in LT. Of note, Treg-cell accumulation in tissues where active infectious processes occur was also documented in experimental models of viral or parasitic infections,35 as well as in human infections.36-38 An important question is whether Treg-cell accumulation in LT represents a feedback mechanism to limit immune-mediated damage, in the context of massive immune activation. In our study, the number of CD69+ T cells, used as a marker reflecting the activation status of the LT, was not correlated with the number of FOXP3+ cells present in the same tissues (Figure 1). Furthermore, similar increases in IFN-γ mRNA expression were observed in LT from progressors and nonprogressors, despite the fact that Treg-cell accumulation occurred only in progressors. Therefore, the persistence of numerous Treg cells in LT of progressor hosts cannot be interpreted solely as a direct consequence of persistent immune activation during the chronic phase of HIV infection. Our in vitro data provide an alternative mechanism, namely that HIV-CD4 interactions can directly drive Treg-cell accumulation through increased Treg-cell survival, explaining why infected hosts with low viral loads exhibited low number of FOXP3+ cells.
Such HIV-mediated effect on Treg cells is likely to involve HIV gp120, as sCD4 blocked FOXP3 induction. Furthermore, engagement of CD4 appears sufficient to drive the process. HIV gp120 is present at high concentrations in LT39,40 and circulates in the blood of HIV-infected donors on virions and as a free protein.41 Although the actual concentrations of gp120 in LT are essentially unknown, they are predicted to be several log higher than in plasma, thus justifying the dose of AT-2 HIV used in this study, which is higher than the reported gp120 plasma levels.42 Of note, both the X4 HIVMN and the R5 HIVADA induced increases in FOXP3 expression. Therefore, the mechanisms described here are not likely to be limited by coreceptor usage.
Our data strongly suggest the hypothesis that CD4-gp120 interactions mediate increased survival of Treg cells, and not conversion of non-Treg cells into Treg cells, based on 2 findings: depletion of CD4+CD25+ T cells before exposure to HIV abolished increased FOXP3 expression; and decreased percentage of apoptotic cells was found in HIV-exposed Treg cells compared with control Treg cells (Figure 6). The mechanisms underlying such increased survival are not identified but could implicate gp120-mediated induction of the MAP kinase Erk,43,44 which in turn would lead to Bad phosphorylation on Ser112.45 Of interest, it was recently shown that, in the presence of IL-2, human Treg cells survive better than effector cells, this effect being mediated by induction of Bcl-X(L) expression and Bad phosphorylation.46 This finding suggests a potential synergic effect of IL-2 and HIV in our in vitro model. TGF-β1 has been described as promoting Treg-cell survival/expansion in vivo,34 as well as the conversion of non-Treg cells into Treg cells in vitro.47,48 However, the fact that no significant induction of TGF-β1 was detected in in vitro HIVexposed CD4+ T cells argues against TGF-β1 as an important survival mechanism in our model.
Of importance, our data show that the functionality of HIV-exposed Treg cells was not altered, on a per-cell basis, in agreement with the fact that circulating Treg cells purified from most HIV-infected patients are functional.16-19 The only qualitative difference between Treg cells from progressor, nonprogressor, and uninfected individuals was the low coexpression of CD25 by FOXP3+ cells in the progressors. Such data are reminiscent of murine models, in which in vivo expansion of Treg cells led to functional Treg cells expressing low levels of CD25, but high levels of CTLA-4 and FoxP3.49 Taken together, our data suggest that the major difference between progressor and nonprogressor individuals is quantitative (ie, the number of Treg cells) and not qualitative.
In contrast to our results, increased cell death of in vitro HIV-infected Treg cells, compared with non-Treg cells (4% vs 1.5%, day 3 after infection) was reported.50 However, the fact that high numbers of Treg cells in LT were still found 2 weeks after peak SIV infection argues against massive killing of Treg cells by virus infection.21 Furthermore, the recent report of Treg-cell accumulation in the gut of untreated HIV-infected patients51 also argues against increased killing of Treg cells in compartments in which viral replication is raging. In addition to the direct survival signals given through HIV gp120–CD4 interactions, TGF-β1 could also promote Treg-cell survival in vivo.52 Of interest, we found increased levels of TGF-β1 in LT from HIVprogs compared with HIVnps and uninfected individuals, although the cellular source of such elevated TGF-β1 was not determined and could be either HIV-infected cells or multiple uninfected cell types activated by the associated inflammatory response.21,53 In contrast, it is difficult to predict the effect of infectious virus on Treg cells. FOXP3 overexpression in HEK293T and purified CD4+ T cells results in suppression of HIV-1 promoter transcription.54 It is therefore possible that Treg cells exposed to infectious HIV undergo limited HIV-1 transcription, which would protect them from HIV-mediated cell death in vivo. More work is clearly needed to address the role of different viral factors in the dynamics of Treg cells in HIV infection.
As markers associated with Treg-cell activity can be up-regulated briefly during the activation of non-Treg cells, it is possible that in vitro and in vivo up-regulation of FOXP3, GITR, and CTLA-4 may also derive from the presence of activated non-Treg cells. However, our current and previous results do not favor this possibility because FOXP3+ cells in the tonsils of HIV-infected individuals did not express CD69,20 and increased in vitro expression of all those markers was not accompanied with concomitant expression of CD69 (Figure 5), arguing against increased FOXP3 and CTLA-4 in vivo and in vitro as markers of non-Treg-cell activation. Furthermore, it was recently reported that although FOXP3 expression is increased in non-Treg cells following anti-CD3/CD28 activation, such induction is temporary, peaking at 48 hours,55 which makes it concomitant to CD69 up-regulation. It should also be noted that several studies have not found increased FOXP3 expression in activated human CD4+CD25– cells.36,37,48
A key question that remains unanswered is how Treg cells mediate immune suppression during HIV infection. CTLA-4 is expressed by a majority of Treg cells in HIV-infected individuals, and has been implicated in the mediation of their suppressive activity. Treg cells may act directly on effector T cells through binding of CTLA-4 to B7 molecules on activated effectors; alternatively, CTLA-4+ Treg cells induce increased expression of the tryptophan-catabolizing enzyme IDO in APCs.15 In agreement with an involvement of the latter pathway, higher IDO expression was evident in progressors compared with nonprogressors (Figure 2). The central role of Treg-cell-APC interactions in Treg-cell function is supported by the fact that in vivo persistent Treg-cell-APC interactions precede the inhibition of effector cells and, conversely, no direct Treg-cell–effector cell contacts are visualized.56 In contrast, Treg-cell-mediated suppression also occurs in APC-free conditions,57 indicating that Treg-cell function may extend beyond APCs. FOXP3+ Treg cells themselves express high levels of IDO during acute SIV infection.21 However, our results do not support this hypothesis in chronic HIV infection, since most IDO+ cells in LT also expressed APC markers.20 Although the suppressive activity of circulating Treg cells from HIV-infected patients was reported to be independent of TGF-β1 or IL-10,17,18 those cytokines may play a role in vivo, as shown in several murine models52,58,59 and in human malaria.60 TGF-β1 was overexpressed in the HIVprog group, indicating a potential role for TGF-β1–suppressive effect. In contrast, IL-10 levels were comparable in progressors and nonprogressors.
Similar expansion of CD8+ lymphocytes and increased IFN-γ expression were found in the LT of progressors and nonprogressors. In contrast, significantly increased perforin expression was found only in LT of SIVnps. These results are in agreement with a previous study,61 showing that HIV-specific CD8+ T cells from nonprogressors, but not from progressors, produce perforin upon restimulation. However, the same difference was not apparent in the studied HIV-infected individuals. This may be due to the fact that most HIVnps included in our study exhibited persistently undetectable viremia (< 50 copies/mL), whereas the threshold of detection of SIV viremia was higher (< 2000 copies/mL). Supporting that argument, the individual with the highest perforin expression (∼ 10-fold up-regulation compared with median expression in uninfected controls) was an HIVnp with detectable viral load at the time of the biopsy. Strikingly, the ratio of expression of perforin to FOXP3 was clearly higher in nonprogressors than in progressors. Since Treg cells interfere with the generation of perforinexpressing CTLs in both murine Friend virus and human hepatitis C infection,62,63 accumulation of Treg cells in LT could constitute an underlying mechanism for inefficient CD8 function in progressor hosts, and consequently, a mechanism involved in progressive disease. In line with this finding, a high intratumor CD8/Treg-cell ratio is a strong predictor for favorable prognosis in ovarian cancer patients.64 Decreased perforin expression in HIVprogs was reported to be more specific of HIV-specific CD8+ T cells, compared with cytomegalovirus (CMV)–specific cells, but not to Epstein Barr virus (EBV)–specific cells.65 These data argue against a Treg-cell-mediated effect, since Treg cells have been described to act in an antigen-independent manner.66,67 However, in vitro depletion of Treg cells from the peripheral-blood mononuclear cells (PBMCs) of HIV-infected patients showed a more pronounced effect on IFN-γ expression by HIV-specific CD8+ T cells, compared with that of CMV-specific cells.16 These data suggest the existence of subtle differences in the susceptibility of different CD8+ subsets and/or of different CD8-mediated functions, to Treg-cell activity, which needs to be carefully examined.
It should be noted that our data do not provide an answer to the important question of whether low numbers of Treg cells in the lymphoid organs of nonprogressor hosts are directly implicated in the differential outcome of infection or are a consequence of low levels of viral replication. Addressing such question will necessitate a prospective longitudinal study of the numbers of Treg cells in lymphoid organs of infected hosts, as well as in vivo studies in which the number/function of Treg cells are manipulated at different stages of infection. Also of note, although our in situ analyses reveal clear differences in the level of expression of Treg-cell markers in relation to HIV disease, they do not constitute a definitive demonstration of the suppressive role of these cells in vivo. This will require detailed functional analyses of LT Treg cells from patients at different stages of HIV infection.
In summary, our data suggest a model in which interactions with HIV drive the accumulation of functional Treg cells in LT. In turn, increased numbers of these cells negatively affect the immune control of virus replication, providing an underlying mechanism for the relationship between Treg-cell numbers and disease prognosis. These findings may have potential for the development of novel therapeutic approaches aimed at enhancing immune function in HIV-infected patients through the manipulation of Treg-cell numbers and/or function.
Authorship
J.N., A.B., P.A.V., R.Z., and C.C. performed research; M.V. and G.F. provided new reagents or analytical tools; J.N., A.B., P.A.V., G.M.S., J.A., and C.C. designed research, analyzed data, and wrote the paper. J.N., A.B., and P.A.V. contributed equally to this work.
The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 10, 2006; DOI 10.1182/blood-2006-05-021576.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The authors thank Dr J. D. Lifson and J. Bess for providing AT-2 HIV preparations, and Dr L. Wahl for providing purified cell populations from HIV-uninfected donors. We also thank Drs C. L. Karp and H. G. Ljunggren for their critical reading of this paper. We also thank all the patients involved in this study. We are also grateful to Drs A. Sönnerborg and C. Broström (Infectious Disease Clinic, Karolinska University Hospital) for their involvement in the recruitment and follow-up of the included patients.
This work was supported by grants from the National Institutes of Health (AI068524 to C.C.); the Swedish Foundation for Strategic Research, the Swedish Cancer Foundation, and the Swedish Research Council (J.A.); and Colciencias (P.A.V.). This research is also supported by the Intramural Program of the Centers for Cancer Research, NCI, NIH (G.F. and G.M.S.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal