Abstract
A control of aspirin response is one proposed strategy for an improvement of anti-platelet therapy. Different methods have been proposed for this indication. We evaluated the prevalence of aspirin non-responsiveness in two different cohorts using a new monitoring method.
Methods: Platelet function was determined using multiple electrode aggregometry (MEA) on the Multiplate analyzer (Dynabyte, Munich, Germany). This device uses a single use test cell with two separate impedance sensors, consisting of a total of 4 electrodes and has 5 channels for parallel tests. Aggregation was triggered using arachidonic acid (0.16 mM, ASPItest, Dynabyte). For the analysis 300 μl of blood are analyzed with the addition of 300 μ saline. Aggregation was quantified by the area under the curve in arbitrary U (1 U corresponds to 10 AU*min). Following IRB approval venous blood was collected from 120 blood donors without anamnestic intake of Aspirin in the 2 weeks before the analysis, in 76 outpatients taking aspirin 25–300 mg/d (mean 128 mg/d) and 341 cardiovascular in-patients on Aspirin 100 mg qd using the direct thrombin inhibitor Melagatran in a final concentration of 15 μ as the anticoagulant.
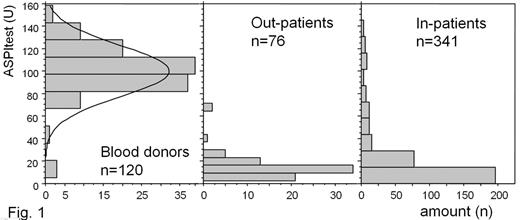
Results: The distribution of the aggregation values is shown in Fig. 1. The median (min–ax) was 100 (5–159) for the blood donors, 13 (2–71) for the out-patients and 11 (0–145) for the in-patients. When a cut-off of 48 (lower end of a 95% confidence interval for the MEA results of the blood donors) is selected, then 51 of 341 in-patients (15%) would have been stratified as non-responders to the aspirin treatment and 2 of 76 out-patients (2,6%).
Discussion: The comparison of the results of aspirin-treated patients and healthy controls (blood donors) reveals a high sensitivity of the new method for the effect of aspirin. Due to the use of a direct thrombin inhibitor as the anticoagulant in our study platelet function was determined under physiological levels of ionized calcium. The results of the two aspirin-treated cohorts examined shows a similar distribution of aggregation values with an aggregation of less than 30 U in 96% of the out-patients and 80% of the in-patients. Using the cut-off of 48 U (based on the results of the blood donors) more patients would have been stratified as aspirin-resistant in the in-patients compared to the out-patients. The out-patients were members of a cardiovascular sports group and knew long in advance that their platelet function would be analyzed on the particular day. Therefore a high level of compliance in respect to the aspirin intake during the days preceeding the analysis can be expected. In addition the out-patient group was significantly healthier compared to the in-patients.
In conclusion multiple electrode aggregometry showed a high sensitivity for aspirin. The rate of non-response to the treatment seems to be influenced by compliance and comorbidities. Prospective trials are required to prove that aspirin-non-response in this particular method is associated with an increased occurrence of arterial thromboembolism.
Disclosures: Andreas Calatzis is a coinventor of the presented method and a coowner of Dynabyte medical, the manufacturer of the instrument.; The study was supported by the manufacturer of the instrument (Dynabyte medical, Munich).
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal