Abstract
Background & Aim: The current recommended starting dose of enoxaparin in children is 1 mg/kg/dose administered twice-daily. Studies in adults, however, have demonstrated similar safety and efficacy between once-daily enoxaparin (1.5 mg/kg/dose) and twice-daily (1 mg/kg/dose) in the initial and long-term treatment of venous thromboembolism. Due to known differences in heparin clearance between adults and young children, recommendations for once-daily enoxaparin dosing should not simply be extrapolated from adult literature. Our objective was to perform a pilot study to describe the pharmacodynamics of once-daily enoxaparin in children, and to gather preliminary data regarding safety and feasibility.
Methods: 15 patients, ages 3 months to 16 years, with newly diagnosed thromboembolism were enrolled. All began therapy with 1.5 mg/kg enoxaparin administered once-daily. Initial peak anti-Xa levels were drawn 4–6 hours after the second dose of enoxaparin. Dose adjustments were made according to a study nomogram to achieve peak anti-Xa levels between 1–2 U/ml, as recommended by the American College of Pathologists for once-daily enoxaparin dosing. Once therapeutic levels were achieved, a subgroup of 8 patients underwent 24–hour pharmacodynamic (PD) monitoring, with anti-Xa levels drawn at Hours 0, 2, 4, 8, 12, 18, and 24.
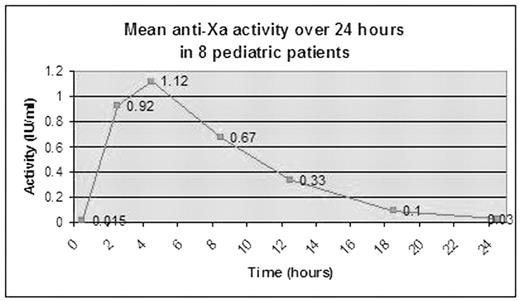
Results: 14 patients completed the study. 8 of 14 children required a final dose of >2 mg/kg to achieve target peak anti-Xa levels (Table). Only one participant (age 15 years) achieved target levels with the original dose. In PD studies, 7 of 8 children had subtherapeutic anti-Xa levels by Hour 12, and 4 of 8 had unmeasurable levels by Hour 18 (Figure). These results differ from adult studies in which therapeutic levels (0.5–1.0 U/ml) have been measurable 13–18 hours after administration, and prophylactic levels (0.1–0.3 U/ml) have been measurable at 24 hours. There were no new or recurrent thrombotic events. One patient was removed from the study on Day 2 because anticoagulation was permanently discontinued after a post-surgical bleed (anti-Xa 0.23 at time of event).
Conclusion: Our pilot data suggests faster clearance of once-daily enoxaparin in the pediatric population as compared to adults. The appropriate starting dose of once-daily enoxaparin in young pediatric patients is likely higher than 1.5 mg/kg.
Dose adjustments needed to achieve target peak anti-Xa activity (1–2 IU/ml)
| Patient . | Age . | Weight (kg) . | Indication for enoxaparin . | Dose changes . | Final dose (mg/kg) . |
|---|---|---|---|---|---|
| DVT, deep venous thrombosis; CVL, central venous line | |||||
| 1 | 3 months | 6.5 | DVT with CVL | 4 | 3.8 |
| 2 | 10 months | 10 | DVT with CVL | 4 | 4 |
| 3 | 14 months | 13 | DVT with CVL | 2 | 2.3 |
| 4 | 2 years | 14 | DVT with CVL | 4 | 2.7 |
| 5 | 3 years | 14 | DVT | 2 | 2.7 |
| 6 | 8 years | 83 | DVT with CVL | 1 | 1.7 |
| 7 | 9 years | 37 | Arterial ischemic stroke | 1 | 1.9 |
| 8 | 13 years | 67 | DVT with CVL | 2 | 2.1 |
| 9 | 14 years | 65 | DVT with CVL | 2 | 2.2 |
| 10 | 14 years | 68 | DVT | 1 | 1.8 |
| 11 | 15 years | 60 | DVT with CVL | 1 | 1.9 |
| 12 | 15 years | 60 | Arterial ischemic stroke | 1 | 1.6 |
| 13 | 15 years | 77 | DVT with CVL | 0 | 1.5 |
| 14 | 16 years | 45 | DVT | 3 | 2.9 |
| Patient . | Age . | Weight (kg) . | Indication for enoxaparin . | Dose changes . | Final dose (mg/kg) . |
|---|---|---|---|---|---|
| DVT, deep venous thrombosis; CVL, central venous line | |||||
| 1 | 3 months | 6.5 | DVT with CVL | 4 | 3.8 |
| 2 | 10 months | 10 | DVT with CVL | 4 | 4 |
| 3 | 14 months | 13 | DVT with CVL | 2 | 2.3 |
| 4 | 2 years | 14 | DVT with CVL | 4 | 2.7 |
| 5 | 3 years | 14 | DVT | 2 | 2.7 |
| 6 | 8 years | 83 | DVT with CVL | 1 | 1.7 |
| 7 | 9 years | 37 | Arterial ischemic stroke | 1 | 1.9 |
| 8 | 13 years | 67 | DVT with CVL | 2 | 2.1 |
| 9 | 14 years | 65 | DVT with CVL | 2 | 2.2 |
| 10 | 14 years | 68 | DVT | 1 | 1.8 |
| 11 | 15 years | 60 | DVT with CVL | 1 | 1.9 |
| 12 | 15 years | 60 | Arterial ischemic stroke | 1 | 1.6 |
| 13 | 15 years | 77 | DVT with CVL | 0 | 1.5 |
| 14 | 16 years | 45 | DVT | 3 | 2.9 |
Figure
Disclosures: The use of enoxaparin in the pediatric population is not FDA-approved. The authors obtained permission from the FDA (IND application 71,971) prior to performing this research study.; Hemophilia and Thrombosis Research Society; General Clinical Research Center, Children’s Hospital of Pittsburgh (5M01 RR00084).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal