Abstract
Background: Targeting the tumor microenvironment through modulation of cytokines is a potential way to treat B-CLL. Thalidomide (THAL) is an immunomodulatory agent with pleiotropic activities, reported to exert antitumor activity through downregulation of cytokines. THAL may enhance proapoptotic activity of fludarabine (F) with resultant improvement of clinical responses. This preliminary study assesses the efficacy and safety of THAL+F combined therapy in B-CLL patients with focus on immune responses.
Patients and methods: Twelve patients (pts) were included in this study. Seven of them (5M, 2F) were newly diagnosed and 5 (2M, 3F) pts were refractory (ref) or relapsed (rel). The median age was 59, range 50–72 yrs. The increased Zap70/CD38 expression had 7/3 pts respectively. All pretreated pts had short lymphocyte doubling time < 6 months. Median prior lines of therapy were 3, range 2–4.
THAL 100 mg po, alone was given for the first 7 days of cycle 1 and continued a la longue for 6 months, F 25 mg/m2 iv, was given for 5 days every 28 days for up to 6 cycles, starting on the seventh day after initiating THAL. Acetylsalicylic acid 75 mg was used to prevent venous thromboembolism. Cytokine levels of TNF, IL-2, IL-4 and IL-10 were assessed before, after THAL and after F treatment (D0, D7 and D12) using ELISA test. T regulatory cells were identified as CD4+CD25highFOXP3+ and assessed by FACS analysis.
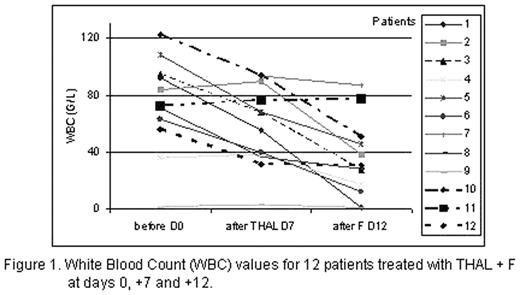
Results:. Median duration of follow-up was 5.5, range 2–7 months. Pts completed 1–5 cycles (mean: 3). In 3 heavily pretreated pts we stopped therapy due to disease progression. Other 9 pts achieved partial remission (7 pts) or stabilization of disease (2 pts). Directly after 1 cycle of therapy 11 pts showed reduction in WBC (Fig. 1). One patient with anemia was transfusion dependent and after 6 weeks of THAL became transfusion free. Interestingly, we have observed increasing levels of TNF during the treatment. Additionally increase in IL-2, IL-4 and IL-10 was observed. We noted significant reduction in absolute count of Treg cells after treatment with THAL (in 9 evaluated patients), that was enhanced in 7 by addition of F to the treatment (mean cells/mL: 315 before treatment, 100 after THAL and 81 after F).
Toxicity: A flare reaction with tenderness and rash of involved lymph nodes was observed during the first cycle in 3 pts. Moderate side effects including constipation and fatigue were noted in all patients. No of them required the additional therapy or discontinuation of THAL+F regimen. Granulocytopenia with WHO grade 4 after the first 2 cycles was noted in 1 patient. Inflammatory complications of respiratory tract with WHO grade 2/3 occured in 3 pts following the first courses of THAL+F.
Conclusions: Results of our study suggests that THAL combined with F demonstrated significant efficacy in B-CLL patients not only as a first line treatment but also as a salvage therapy with good safety profile. THAL+F regimen should be tested on a larger group of patients.
White Blood Count (WBC) values for 12 patients treated with THAL + F at days 0, +7 and +12.
White Blood Count (WBC) values for 12 patients treated with THAL + F at days 0, +7 and +12.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal