Abstract
Objective: To describe the use and results of imatinib mesylate (IM) treatment for chronic myeloid leukemia (CML) in the Nord-Pas de Calais Region in Northern France (~ 4 millions inhabitants).
Methods: we identified all IM treated patients (pts) within the population of all confirmed diagnosis of CML occurred during the period 1985–2004. IM resistance (haematological and cytogenetic imatinib failure and suboptimal response) was evaluated according to the European LeukemiaNet consensus.
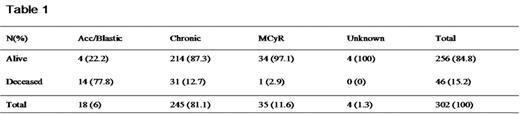
Results: 302 pts (38.6% of the global cohort of diagnosis) were included in this retrospective study. If we consider new diagnosis occurred after Januar 1st 2001, 163 pts (90.6%) have been treated by IM. At the initiation of IM: 18 pts (6%) were in advanced phase (accelerated and blastic), 245 pts (81,1%) in chronic phase (CP) (with Ph+ metaphases > 35%), 35 (11,6%) had major cytogenetic response (MCyR) obtained with interferon (IFN) or bone marrow transplantation (BMT). Four pts (1,3%) had an unknown status. The percentage of alive pts at the last update (December 31th 2005) according to the disease stage at initiation of treatment is given in the table 1.In the sample of 245 CP pts, 139 (56.7%) had been previously treated by IFN, the median CML duration prior to IM was 4.7 months [0–174], the median IM treatment duration was 31.3 months [0.5–72.3] and the median follow-up after IM initiation was 36 month [5.2–72.3]. 214 pts (87.30%) were alive at the last update with 169 (79%) still on IM and 161 (75.2%) on IM as monotherapy. The median and mean IM daily doses of alive pts are 400 mg and 409.5 mg, respectively. Seventeen pts (6.9%) stopped IM for intolerance. The haematological and cytogenetic follow-up was informative for 206 CP pts (84.1%): MCyR 155 pts (75.2%) and CCyR 144 pts (69.9%). IM resistance occurred in 73 (35.4%) of these 206 evaluable pts, including 23 (11.1%) accelerations and blastic transformations. In the subgroup of IM resistant pts 47 (64.4%) have increased their IM posology. The mean of the maximum daily dose in the resistant subgroup was 607.5 mg (median 600 mg). BCR-ABL kinase domain mutations were found in 15 (20.5%) pts amoung IM resistant population. The estimated 5-years survival rate of the CP pts is 81.3%. Previous treatment by IFN doesn’t influence significantly the survival, nor the duration of the disease before IM initiation, even if we found a trend for a better survival when this period was inferior to 6 months (5 years survival rate 90.4% vs 77.5%, p=0.16). BMT was performed for 31 pts (10.3%) including 19 pts (6.3%) after the initiation of IM therapy.
Conclusion: these real life observations show the large use of IM in the last years and its great impact on the management and the survival of CML pts. IM resistance occurs in 1/3 of pts but the disease duration prior to IM is heterogeneous. An increase of IM dose has been tried in most IM resistant pts. The response to the different therapy adaptations is under study and will be presented. The vast majority of pts are still on IM therapy at the last update.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal