Abstract
Imatinib mesylate has substantially changed treatment of chronic myeloid leukemia (CML). But the resistance-conferring mutations in tyrosine KD (Kinase Domain) of BCR-ABL region constitute the leading cause of clinical resistance in CML patients on imatinib or dasatinib monotheray. Therefore early detection and accurate quantification of the common mutations in KD in post-transplantation patient could provide vital information regarding disease progression or prognosis. Here we report results of pyrosequencing to quantify the non-mutated and mutant alleles in 11 CML patients who were treated by imatinib mesylate or dasatinib. Patients were monitored over periods ranging from 24 to 67 months. 11 mutations in the region identified by direct or pyrosequencing and/or pGEMT cloning are Y215F, T253F, E255K, L323P, N336S, A337T, M351T, E355K, F359I, H396R and P480L. Out of 11 mutations, two (F359I and P480L) are found to be novel. Four mutations (L323P, N336S, A337T and E355K) were positioned between P loop and catalytic domain, one (F359I) was in the activation domain and one (P480L) was near to the carboxy terminal of the kinase. One novel mutation (Y215F) located outside (N terminal region of kinase domain) region was also identified. Y215F mutation which lies outside the kinase domain might be sporadic one without affecting kinase activity. We successfully measured proportion of KD mutant cells with greater precision by using pyrosequencing (Fig 1). The dynamics of the mutated clones obtained from pyrosequencing were correlated with RQ-PCR (Fig2) suggesting quantitative pyrosequencing method could be alternative to various methods including allele-specific oligonucleotide-polymerase chain reaction (ASO-PCR) and conventional sequencing which have limited sensitivity at low concentrations of mutant allele. Taken together, we established a novel method for early identification of KD mutations and precise monitoring of the proportion of mutant alleles in patient with CML. Most importantly, those results were in correlation with clinical response to imatinib mesylate or dasatinib. Therefore quantitative measurement of mutant cell dynamics could provide early indications of clinically significant disease progression.
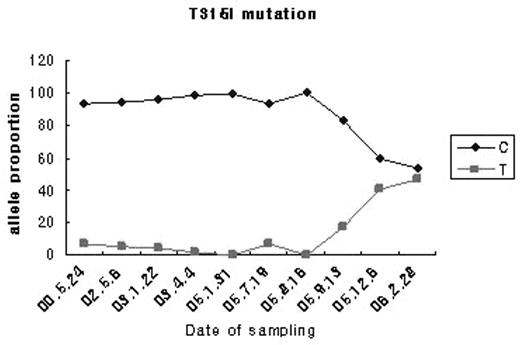
T3151 mutation
T3151 Mutation
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal