Abstract
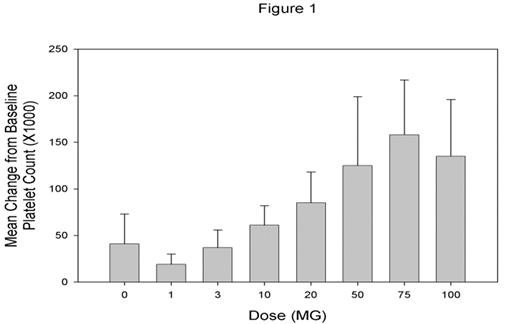
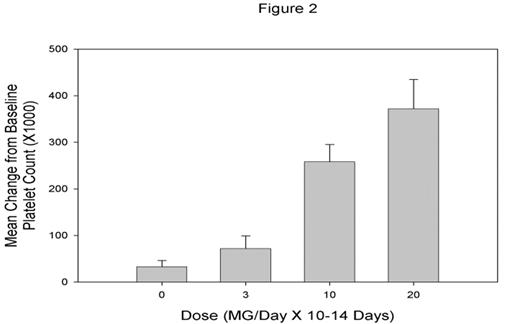
AKR-501 is an orally active thrombopoietin (TPO) receptor agonist that increases platelet production through stimulation of megakaryocyte proliferation and differentiation. The effect of AKR-501 in in vitro and in vivo models is additive with that of rhTPO. AKR-501 is a potential new therapy for use in the prevention or treatment of thrombocytopenia of various etiologies. We evaluated the safety, pharmacokinetics and pharmacodynamic activity of AKR-501 when administered as single and multiple oral doses to healthy volunteers. In the single dose study, 7 dose cohorts of 9 subjects each were randomized in a ratio of 2:1 to receive increasing doses (1, 3, 10, 20, 50, 75 and 100 mg) of AKR-501 or placebo. In the multiple dose study, 5 dose cohorts of 9 subjects each were to be randomized in a ratio of 2:1 to receive increasing doses (3, 10, 20, 50 and 100 mg) of AKR-501 or placebo administered daily for 14 days. All subjects were evaluated for safety and pharmacodynamic activity as assessed by peripheral blood platelet counts (PBPC). Dose escalation was stopped on Day 10 of dosing in the 3rd dose cohort (20 mg/day) when 6 of the volunteers exhibited a platelet count above 500,000/mL. The protocol defined endpoint of >50% increase over baseline platelet count was achieved in 5 of 6 volunteers given a single dose of 100 mg, and in all 6 volunteers given daily doses of 10 mg for 14 days and 20 mg for 10 days. The drug was well tolerated in both the single and multiple dose studies with no serious drug-related adverse experiences reported at any dose. AKR-501 was consistently well absorbed exhibiting dose linear pharmacokinetics following single and multiple dose administration, and a serum half-life of approximately 16 hours. The mean observed Cmax following administration of single doses increased from 5.67 ng/mL at 1 mg to 388 ng/mL at 100 mg. The mean observed AUC increased from 174 ng·hr/mL at 1 mg to 11052 ng·hr/mL at 100 mg. Accumulation to steady state with multiple dosing was consistent with predictions based on a linear model and the single dose PK parameter estimates. There was a linear correlation of individual observed peak activity with corresponding Cmax (single-dose r2=0.70 p<.0001, multiple-dose r2=0.67 p<0.0001). The mean change from baseline PBPC for each dose cohort in the single-dose (Figure 1) and multiple-dose (Figure 2) studies are shown in the following graphs.
Disclosures: Employee of AkaRx Inc. - RD, DT, RL.; Consultant to AkaRx Inc. - DK.; Shareholder in AkaRx Inc. - RD, DT, RL.; Member Board of Directors AkaRx Inc.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal