Abstract
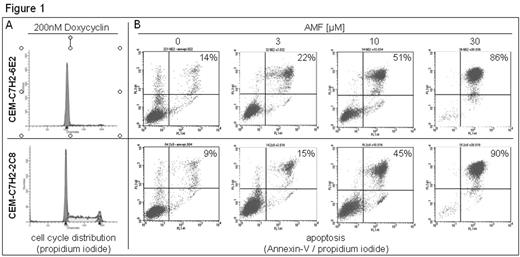
We have previously shown that Akacid-medical-formulation (AMF) exerts its antiproliferative effects on various malignant solid cell lines, including those derived from prostate via downregulation of mitogen-activated protein kinases Erk 1/2 and cell cycle regulators, e.g. cdk-2, -4, cyclin E, -D1 (Neuwirt et al. 2006). However, the effect of AMF on proliferation and induction of apoptosis in malignant hematologic cell lines has not been investigated yet. Therefore, we performed 3H-thymidine incorporation assays to assess the growth inhibition of HL-60, U-937, K-562, CEM-C7H2 cells after treatment for 48 hours with various concentrations of AMF (0.3 – 100 μM). All cell lines were dose-dependently inhibited by AMF with an IC50 of about 2.1μM. As cellular growth arrest is known to be one major reason for resistance to chemotherapeutics, we investigated the effect of AMF on the cell line CEM-C7H2-6E2, in which G1/0-phase arrest can be induced by tetracyclin-regulated expression of the cell cycle inhibitor p16INK4A (Fig. 1A). Flow cytometric analysis using Annexin-V / propidium-iodide staining showed that in G1-phase arrested cells apoptosis was induced to a similar extent (LD50 of 10μM) as compared to proliferating control cells (CEM-C7H2-2C8) (Fig. 1B). In addition, we tried to find out whether AMF induces apoptosis via the caspase-9 dependent intrinsic or the caspase-8 dependent extrinsic pathway. For this purpose, two different cell lines stably transfected with tetracyclin-responsible plasmids encoding for the antiapoptotic proteins bcl-2 (CEM-C7H2-10E1) and CrmA (CEM-C7H2-2E8) were used. After 48 hours of treatment with AMF (1 – 30μM) we found that overexpression of neither bcl-2 nor CrmA could inhibit AMF-induced apoptosis compared to control cells (LD50 15μM). Data on apoptosis obtained by flow cytometry were confirmed by Western blot analysis on activated caspase-8, -9, -3, and cleaved PARP in CEM-C7H2 cells. Caspase-8 and -9 were not activated after 48 hours. However, it is interesting that downstream effectors of apoptosis, cleaved PARP and caspase-3, were strongly induced (1000 % of control at 30μM). In conclusion, our results show a potent antiproliferative effect of AMF on leukemic cell lines. Induction of apoptosis could not be inhibited either by G1-phase arrest or overexpression of the antiapoptotic proteins bcl-2 or CrmA. Although we found a substantial activation of the downstream effectors of apoptosis by AMF including caspase-3 and PARP, the upstream pathway of activation remains unclear.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal