Abstract
The Cooperative Study of Sickle Cell Disease (CSSCD) reported that dactylitis, severe anemia, and leukocytosis in very young children with SCD increased the risk of later adverse outcomes, including death, stroke, recurrent acute chest syndrome (ACS), and frequent pain (
NEJM 2000;342:83
). Specifically, in the CSSCD infant cohort, the occurrence of dactylitis in the first year of life (“early dactylitis”) and a steady-state hemoglobin (Hgb) concentration <7 g/dL in the second year of life (“severe anemia”) together predicted a high risk (0.36 probability) of a later adverse outcome. A steady-state leukocyte count of approximately 20,000/mm3 or higher in the second year of life (“leukocytosis”) in combination with either early dactylitis or severe anemia also predicted a high risk of an adverse outcome. The testing of this model in other cohorts has not been reported, so we evaluated its performance characteristics in the Dallas Newborn Cohort (Blood 2004;103:4023
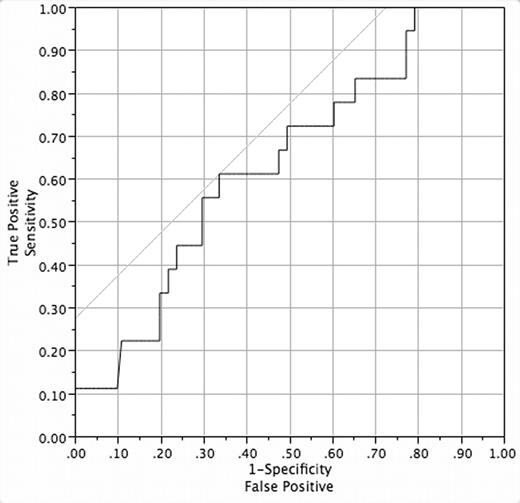
). We studied all cohort members with sickle cell anemia (SS) or sickle-β0-thalassemia (Sβ0) who had 5 or more years of follow-up and complete laboratory data and medical records. We searched our electronic database and patients’ medical records to ascertain the occurrence of the three predictors (early dactylitis, severe anemia, and leukocytosis) and the four adverse events [death; clinically overt first stroke; frequent pain (average of 2 or more painful events/year for the entire follow-up period, with ≥2 events/year for 3 consecutive years); and recurrent ACS (average of 1 or more episode/year with ≥1 episode/year for 3 consecutive years)]. We only considered hospitalizations for pain in this analysis, unlike the CSSCD, because we do not systematically track episodes of pain treated only at home or in outpatient facilities. We studied a total of 119 children (68% male, 97% SS) who had a mean follow-up of 9.7 years (range 5 – 15.4). Twenty-four (20%) had early dactylitis, 5 (4%) had severe anemia, and 15 (12.6%) had leukocytosis. Eighteen subjects (15.1%) experienced adverse events: 2 (1.7%) died, 13 (10.9%) had a stroke, 3 (2.5%) had frequent pain, and 1 (0.8%) had recurrent ACS. Sensitivity and specificity of the model were 0 and 97%. Positive and negative predictive values were 0 and 84%. We used binary logistic regression to further evaluate the model. The whole model test was not significant (P=0.21), and R2 was 0.044. The area under the ROC curve was 0.64 (Figure), and better-performing cut-points for Hgb and leukocyte count could not be found. In summary, the CSSCD early prediction model had very low sensitivity and positive predictive value in the Dallas Newborn Cohort. Specificity and negative predictive value were high, however. This retrospective analysis has several limitations and biases, but it suggests that the CSSCD model does not well predict adverse outcomes in the Dallas Newborn Cohort.Disclosure: No relevant conflicts of interest to declare.
Author notes
*
Corresponding author
2006, The American Society of Hematology
2006

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal