Abstract
The effect of prior exposure to rituximab (ritux) on relapse (REL) and survival following stem cell transplantation (SCT) in patients (pts) with REL composite low and intermediate grade non-Hodgkin lymphoma (L/I-NHL) is unknown. Fifty-four pts with REL L/I-NHL underwent high-dose chemotherapy (CT) and SCT Jan ’89 to June ’05. Follow-up is complete to April ’6 with a median of 32 mo. Ritux was added to CT regimens since 2001 and given at 375 mg/m2. Eighteen pts received ritux at initial diagnosis of NHL or after REL with salvage CT prior to SCT. Pts proceeded to high dose CT with allogeneic (allo) (n=12) or autologous (auto) SCT (n=6). This group was compared with a group of pts not receiving ritux pre-SCT (n=36)(Table 1).
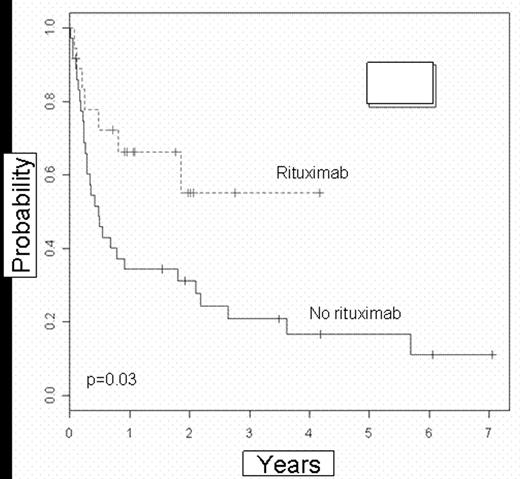
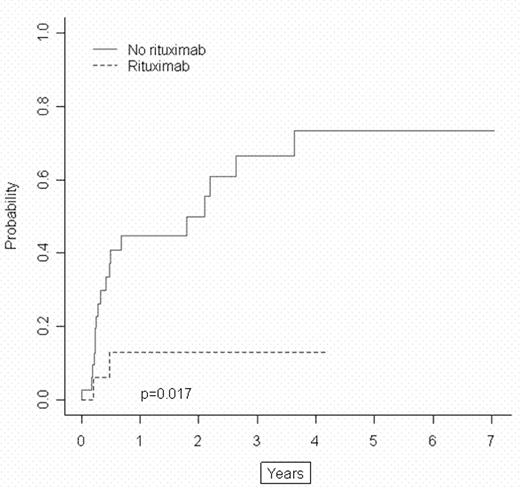
Eleven pts (61%) are alive in the ritux group compared with 11 pts (31%) in the non-ritux group. The 2 and 4-y OS for pts who received ritux were 52% and 52%, vs 36% and 24% (p=.12, RR=.52) for pts who did not receive ritux. The EFS were significantly different with 2 and 4-y EFS for the ritux group 56% and 56% vs 24% and 18% (P=.038, RR=.42) for the non-ritux group (figure 1). No effect was seen on TRM. The risk of REL post SCT was significantly lower in the ritux group (p=.017)(figure 2). Two of 18 pts (11%) had NHL REL in the ritux vs 18 of 36 (50%) in the non-ritux group. The hazard rate of REL in pts who received ritux was 20% that of pts in the non-ritux group. Significance maintained in multivariate analysis (p=.016).
No impact was seen on graft-versus-host disease (GVHD). Fifty percent and 61% of pts developed acute GVHD grades 2–4 and 50% and 64% of pts developed chronic GVHD in the ritux and non-ritux groups respectively.
In conclusion, prior treatment with ritux in pts with relapsed composite L/I-NHL undergoing SCT was associated with reduced risk of REL and improved survival without associated increase in toxicity. Further analysis is underway to clarify the nature of this important finding.
Clinicopathological characteristics of ritux and non-ritux groups
| Parameter$ . | Rituximab group, n=18 (%) . | Non-rituximab group, n=36 (%) . |
|---|---|---|
| $ all p values are >0.1. *at diagnosis | ||
| Median age at SCT | 48 y | 44 y |
| M:F | 2.6:1 | 1.6:1 |
| Allo-SCT | 12(67) | 28(78) |
| BM involvement* | 10(56) | 22(61) |
| B symptoms* | 7(39) | 10(28) |
| Prior purine analogue therapy | 8(44) | 11(31) |
| Residual disease prior to SCT | 9(50) | 14(39) |
| TBI in conditioning | 13(72) | 30(83) |
| Parameter$ . | Rituximab group, n=18 (%) . | Non-rituximab group, n=36 (%) . |
|---|---|---|
| $ all p values are >0.1. *at diagnosis | ||
| Median age at SCT | 48 y | 44 y |
| M:F | 2.6:1 | 1.6:1 |
| Allo-SCT | 12(67) | 28(78) |
| BM involvement* | 10(56) | 22(61) |
| B symptoms* | 7(39) | 10(28) |
| Prior purine analogue therapy | 8(44) | 11(31) |
| Residual disease prior to SCT | 9(50) | 14(39) |
| TBI in conditioning | 13(72) | 30(83) |
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal