Abstract
Background: Multiple myeloma remains incurable with current approaches and newer therapies are needed to improve the outcome of these patients. While monoclonal antibody based therapies have been successful in some of the hematological malignancies, such approaches have had limited efficacy in the setting of myeloma. Thymoglobulin (polyclonal rabbit antithymocyte globulin, Genzyme) (Thymo) has been extensively evaluated in the setting of allogeneic blood and marrow transplantation and solid organ transplants. Given the polyclonal nature of this product, with antibodies against different B cell antigens, we evaluated the in vitro and in vivo activity of Thymo in myeloma.
Methods: MM cell lines were cultured in RPMI 1640 containing 10% fetal bovine serum supplemented with L-Glutamine, penicillin, and streptomycin. The KAS-6/1 cell line was also supplemented with 1 ng/ml IL-6. Cytotoxicity following drug treatment was measured using the MTT viability assay. Apoptosis was measured by flow cytometry using Annexin V/PI in cell lines and Apo 2.7 in primary patient plasma cells. Shifts in expression of a variety of different B cell and plasma cell antigens were examined on several different myeloma cell lines following Thymo treatment in order to identify the potential antigenic targets. In vivo activity of thymo was evaluated in a SCID plasmacytoma model injected with RPMI myeloma cell lines.
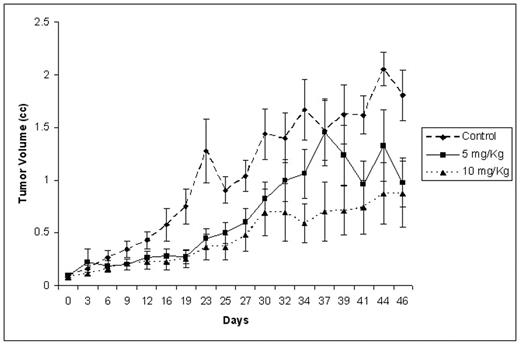
Results: rATG was cytotoxic in vitro to several MM cell lines (RPMI 8226, U266, OPM1, OPM2) including the IL-6 dependent cell line Kas6/1 with LC50 of around 1 mg/mL. Additionally, thymo was cytotoxic MM cell lines resistant to conventional agents such as doxorubicin (Dox40), melphalan (LR5) and dexamethasone (MM1R). Thymo induced apoptosis in MM cell lines and in patient derived primary myeloma cells. When tested in combination with other anti-myeloma agents an additive effect was seen with doxorubicin, PS341 and melphalan. Using competitive flow cytometry, we identified CD138, CD38, Cd45, CD126, CD49d (VLA4), as well as CD20 as antigens likely to be targeted by Thymo. Tumor bearing mice injected with Thymo at two different doses (5 mg/kg and 10 mg/kg for five days) had significantly delayed tumor growth compared to non-injected mice, and this translated into a better survival for these mice. Mice receiving 10 mg/kg dose had a slower tumor growth compared to 5 mg/kg dose (Figure).
Conclusions: Thymoglobulin has promising in vitro and in vivo activity in the setting of myeloma. These studies will provide the rational for future clinical development of this agent in myeloma alone or in combination with other agents. Based on these results, we are in the process of initiating a clinical trial combining Thymo with Melphalan.
Disclosures: Funding from Genzyme to SK for laboratory research.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal