Abstract
AGVHD is a life threatening complication of allogeneic HSCT, initiated by host and donor DC stimulation of donor T lymphocytes. Current AGVHD prophylaxis targets T lymphocytes, compromising anti-viral and therapeutic anti-leukemic responses. The presence of activated blood DC predicts for clinical AGVHD and we predict that a new strategy targeting therapy to activated DC should prevent alloimmune induction of AGVHD but preserve protective T cell immune responses. CD83 is a cell surface molecule expressed by activated DC. Having shown that a rabbit polyclonal antibody to human CD83 (RA83) depletes activated DC and suppresses alloimmune reactions in vitro, we tested the effect of RA83 treatment on T cell immunity in vitro and its ability to prevent human PBMC induced xenogeneic AGVHD in vivo.
The effect of RA83 treatment on T cell numbers, proliferation and cytokine secretion in allogeneic MLR was compared with appropriate controls, including Campath-1H. Allogeneic responses in vitro were mirrored in a DC dependent in vivo model of xenogeneic AGVHD, in which 50×106 human PBMC were injected into an irradiated SCID mouse. Human cytokine levels were measured in MLR tissue culture supernatant (TCSN) and mouse serum at the time of sacrifice. Cytotoxic T cell responses to viral antigens (CMV and FMP) were analysed by specific pentamer analysis and Cr51 release assays, prior to and following HLA restricted peptide antigen specific expansion.
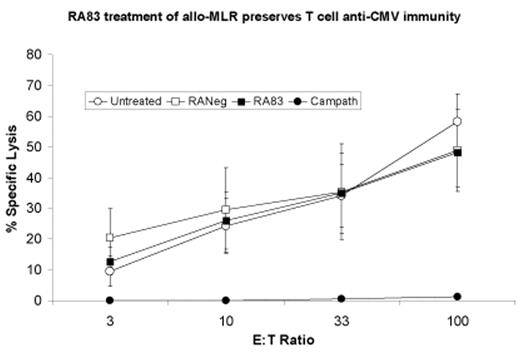
RA83 was shown to have NK-mediated ADCC capacity. Cellular proliferation in the allogeneic MLR was reduced by both RA83 and Campath-1H treatment (p= 0.004 and 0.01 respectively vs controls) and both antibodies improved mouse survival in the human xenogenic AGVHD model (RA83: 93%, Campath-1H: 100%, p<0.0001). IFN-g was significantly reduced in the TCSN from MLR treated with RA83 (p=0.0391) and in sera taken from RA83 (p=0.0002) and Campath-1H (p=0.0051) treated mice. Serum IL-4 levels were maintained in RA83 and Campath-1H treated mice. The serum levels of IL-5, IL-8 and TNF in mice treated with RA83 were markedly reduced compared to controls (p=0.0256, 0.0025, 0.025, respectively), and the reductions were similar to those seen in Campath-1H treated mice. Similar numbers of T cells were recovered from RA83 treated and control MLR, and both CMV and FMP specific CD8+ T cells were retained. These cells were readily expanded by peptide pulsing and autologous restimulation and had specific cytotoxic activity comparable to control cultures (see figure). In contrast, Campath-1H treatment removed specific anti-viral responses (vs controls: CMV: p<0.00001 and FMP: p=0.0051).
Specific antibody to CD83 depletes activated DC in vitro and prevents xenogeneic human DC dependent AGVHD in vivo. This was accompanied by a Th1 to Th2 skewing of the cytokine response. RA83, but not Campath-1H treatment retained normal numbers of T cells and maintained normal cytotoxic responses to common post-transplant viral infections. Depletion of activated DC may be an effective means of AGVHD control, which maintains T cell immunity to life threatening infections and potentially anti-leukaemia responses.
RA83 treatment of allo-MLR preserves T cell anti-CMV immunity
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal