Abstract
Background: The posttransplant reconstitution of the αβ TCR repertoire correlates with the control of viral infections. We have previously demonstrated that using a limited number of patients cohort some had a skew of the Vδ1+ TCRγδ+ T cell repertoire after allogeneic HSCT, and that clonal predominance of Vδ1+ TCRγδ+ T cells was already present in blood of some donors and those clones were transferred to the recipients. The proliferation of Vδ1+ γδ T cells has been described in various infections including HIV, CMV and malaria. Although the functions of human Vδ1+ γδ T cells in vivo remain obscure, aforementioned results have raised the hypothesis that Vδ1+ T cells may have the repertoire against microorganisms widely infected in humans.
Objective: We sought to explore the biological role for this T cell subset by investigating the reconstitution of the Vδ1+ γδT cell repertoire after human allogeneic HSCT, and the cytotoxic activity against EBV-infected cells of this T cell subset isolated from the long-term survivor.
Methods: The size distribution and sequences of CDR3 of TCR δ-chains were determined by using an automated DNA sequencer. Immunophenotypes of the cells were analyzed by flow cytometry. PBMCs were stimulated with autologous EBV-LCL in the presence of IL-2 followed by positive selection with a MACS TCRγ/δ MicroBead kit. Positively selected γδ T lymphocytes were cultured with irradiated autologous EBV-LCL and allogeneic PBMCs as feeder cells, PHA (1 μg/ml) and IL-2 (10 U/ml) for 7 days. Vδ1 T cells were enriched by depletion of αβ T cells, Vδ2+ and Vδ3+ T cells with mAbs and an anti-FITC MicroBead kit and LD columns. Cytotoxicity was measured by standard 4-hr 51Cr release assay.
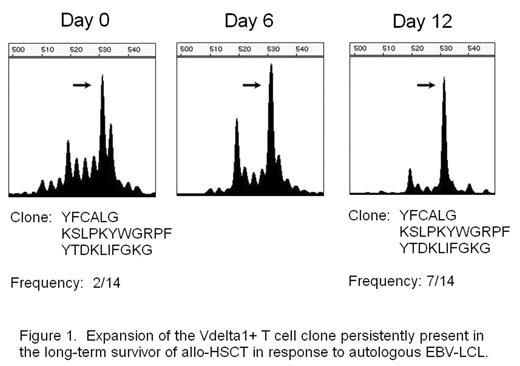
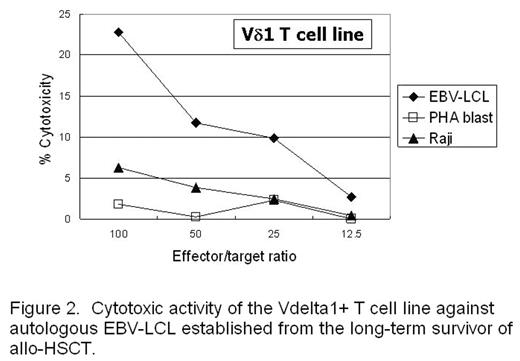
Results: We observed skewed TCR repertoires of the Vδ1+ γδT cells in 27 out of 44 patients receiving allogeneic HSCT. The -WGI- amino acid motif was observed in CDR3 of clonally expanded Vδ1+ T cells in half of the patients. A skew was also detected in certain healthy donors, and the Vδ1+ T cell clone derived from the donor mature T cell pool was persisting in the recipient’s blood even ten years after transplant. In addition, this T cell clone expanded in vitro against the stimulation with autologous EBV-LCL (Fig. 1), and the Vδ1+ T cell line expanded in vitro from the same patient showed cytotoxicity against autologous EBV-LCL (Fig. 2). EBV-infected cells could also induce oligoclonal expansion of Vδ1+ T cells in healthy individuals as well.
Conclusion: The skewing of Vδ1+ γδT cell repertoires may be the result of the responses to latently infectious antigens, and human Vδ1+ T cells may have a role in the protection against EBV infection.
Expansion of the Vdelta 1+ T cell clone persistently present in the long-term survivor of allo-HSCT in response to autologous EBV-LCL
Expansion of the Vdelta 1+ T cell clone persistently present in the long-term survivor of allo-HSCT in response to autologous EBV-LCL
Cytotoxic activity of the Vdelta 1+ T cell line against autologous EBV-LCL established form the long-term survivor of allo-HSCT.
Cytotoxic activity of the Vdelta 1+ T cell line against autologous EBV-LCL established form the long-term survivor of allo-HSCT.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal