Abstract
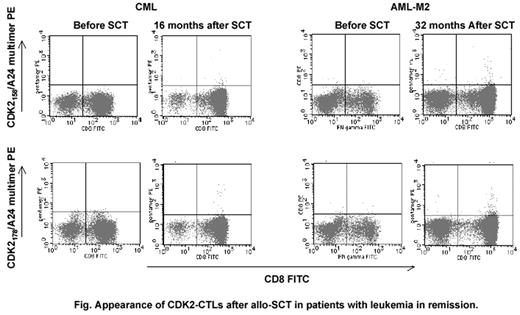
Aberrantly expressed self-antigens in leukemic cells serve as leukemia-associated-antigens (LAAs) of leukemia reactive T cells. Although such self-antigen-derived LAAs potentially induce high avidity CTLs in patients with leukemia, these CTLs usually do not persist due to apoptosis upon encountering leukemic cells. In allogeneic stem cell transplant (allo-SCT) recipients with leukemia, residual leukemic cell may sensitize donor-derived T cells by LAA in vivo and induce high avidity CTLs specific to leukemic cells. Cyclin-dependent kinase 2 (CDK2) is a cell cycle regulator protein that is aberrantly expressed in AML, MDS, ALL and MCL cells. We previously reported that CDK2-derived nonamer peptides (CDK2158: TYTHEVVTL, CDK2167: WYRQPEILL, CDK2178: KYYSTAVDI) avidly bound to HLA-A24 molecule to elicit each peptide-specific CTL from peripheral blood mononuclear cells (PBMCs) of healthy individuals (Blood. 106 (11): a3103. 2005). When we generated CDK2158-specific CD8+ T cells from an HLA identical sibling donor of a patient with AML by stimulating donor PBMCs with CDK2158-coated HLA-A24-transfected T2, CDK2158-specific CD8+ T cells preferentially killed the recipient AML cells which aberrantly expressed CDK2 proteins. The percentages of specific lysis in the 51Cr-release assay at an E/T ratio of 40 were 36.8% for AML cells and 24.8% for autologous PBMCs. To determine if CDK2-specific CD8+ T cells are present in PBMCs from allo-SCT patients, we studied 14 patients possessing HLA-A24 [6 patients with AML (2 AML-M0, 3 AML-M2, 1 AML with multilinage dysplasia), 1 with MDS, 1 with CML, 2 with ALL, 2 with MCL and 2 with RCC] using CDK2158/A24 and CDK2178/A24 multimers. Cryopreserved PBMCs obtained before and 4–73 months after allo-SCT were assayed for multimer staining. The source of graft was unrelated BM in 4, related BM in 2, related PBMC in 3 and CB in 5 patients. All CB and 1 BM graft were HLA mismatched. Seven patients were in complete remission (CR) at the time of SCT while 7 were in non-CR. Ten patients remained in CR 4–79 months after SCT. Small populations (0.13–0.77 % of lymphocyte) of CDK2158 and CDK2178-specific CTL were detectable in 5 patients (3 with AML-M2, 1 with CML and 1 with ALL) 10–73 months after SCT. All of the 5 remained in CR 11–79 months after SCT. Only one of them had active cGvHD at sampling. On the other hand, among 7 patients who did not show an increase in the proportion of neither CDK2158 nor CDK2178-specific CTLs, 2 patients with AML-M0 relapsed 4 and 5 months after SCT, respectively. Both of them had active cGvHD at sampling. CDK2-specific CTLs were also undetectable in 2 patients with RCC after allo-SCT. These data provide evidence for the first time that CDK2-specific CTLs can be induced from donor-derived T cells without peptide immunization possibly due to in vivo sentitization of donor T cells by residual leukemic cells. Vaccination with CDK2-derived peptides after allo-SCT may therefore be useful in inducing CDK2-specific CTLs and thereby preventing relapse of leukemia.
Appearance of CDK2-CTLs after allo SCT in patients with leukemia in remission.
Appearance of CDK2-CTLs after allo SCT in patients with leukemia in remission.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal