Abstract
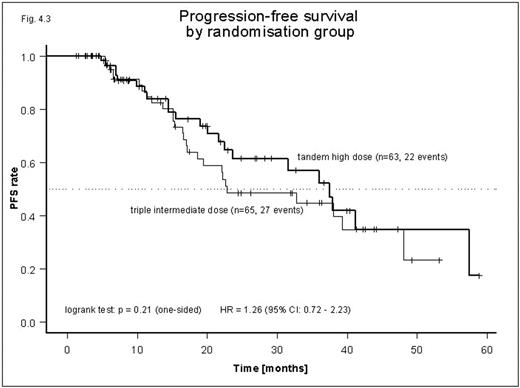
In this study we compare the efficacy and toxicity of double T with M 200 with triple T using M 100 in newly diagnosed pts with MM. 175 pts have as yet been enrolled and 135 have been randomized after VAD induction treatment and stem cell collection to either tandem or triple transplantation. Reasons for non-randomization were too early for T (n=22), PD after induction (n=5), early death (n=3), toxicity (n=3), pt. refusal (n=3), and others (n=4). Median age of the 135 pts randomized was 59 years (range: 27–70 years). Stage I: 8%, stage II: 17%, stage III: 75%, Paraprotein Isotypes: IgG: 55%, IgA: 25%, IgD: 2%, IgM: 1%, Light chain: 17%. Induction treatment: 3 cycles of VAD; stem cell priming: IEV (ifosfamide (2g/m2)-etoposide (150mg/m2), d1–3; epirubicin (50mg/m2), d1) and G-CSF (5mg/kg) from d4. Conditioning regimen: M 200 mg/m2 or M 100 mg/m2 plus G-CSF; day 5 until WBC >2000/ml. Pts with ≥ SD after T were subsequently randomized to interferon 3MU, TIW (Schering Plough) plus prednisone (25mg, po., TIW), or interferon alone. QoL was assessed with the EORTC QLQ C30 instrument. Chi2 test for trend was used for comparison of response and survival endpoints were estimated by the Kaplan-Meier product limit method. Response rates after VAD were CR: 11%, PR: 65%, SD: 22% and PD: 2%. Median time from start of VAD to start of T was 137 in the double and 139 days in the triple T arm. Response rates did not differ between pts treated with double or triple T (CR: 47% vs. 43%, PR: 47% vs. 47%, SD 2% vs. 6%, PD: 4% vs. 4%, p=0.60, respectively). Median PFS for both groups combined was 36 mos, but showed a tendency for shorter PFS in pts on triple T (23 mos. vs. 37.5 mos; HR 1.26, 95% CI: 0.72–2.23, p=0.21). Median of OS has not been reached yet, but did not differ between both groups. Hematological toxicity was similar in both groups. There was a tendency for more grade 2–4 mucositis and for grade 3–4 vomiting, nausea, and fatigue in the double T group. Global QoL (questions 29 and 30) was comparable between both groups, before (5 (2.5–6.5) vs. 5 (4.5–6)) and after double (5.8 (3–8.5) or triple T (6 (2.5–7)). In conclusion, triple T with intermediate dose M resulted in similar response rates, slightly shorter PFS but similar OS and was associated with less mucositis and slightly less vomiting, nausea and fatigue.
Progression-free survival by randomization group
Disclosures: Interferon is started as maintenance treatment for multiple myeloma.; Participation in Advisory Boards: Amgen, Roche, Janssen-Cilag, Schering-Plough.; Schering-Plough.; Amgen, Roche, Janssen-Cilag.; Amgen, Roche, Janssen-Cilag.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal