Abstract
BACKGROUND: Fludarabine-based chemotherapy combinations are highly effective in patients (pts) with low-grade follicular lymphoma (FL), but cause severe and long-lasting immunosuppression due to depletion of normal CD4+ T-cells. Aside from increasing the risk of serious infections, this toxicity may limit the ability of the immune system to eliminate minimal residual disease. Adoptive immunotherapy using autologous CD25-depleted, CD3/CD28-costimulated T-cells (ACTC) expanded ex vivo may enhance immune reconstitution and improve disease control.
METHODS: We initiated a phase I study in pts with purine analog-naive relapsed/refractory FL (grades 1 and 2). After leukapheresis, pts receive 4 cycles of fludarabine (25 mg/m2) days 1–3 and cyclophosphamide (250 mg/m2) days 1–3. Four weeks after the last cycle, responding patients (CR/CRu, PR) receive escalating doses of ACTC prepared ex vivo from autologous T-cellss collected prior to chemotherapy and depleted of regulatory CD4+/CD25+ cells, then expanded and activated using anti-CD3 and anti-CD28.
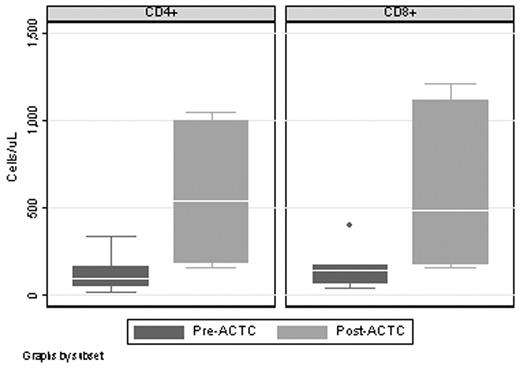
RESULTS: Eleven pts have been enrolled to date. Median age is 49 y (range: 33–64y). Median number of prior therapies is 2 (range: 1 – 3). Two pts were withdrawn from the study due to hematologic toxicity related to chemotherapy, one patient was withdrawn for progressive disease during chemotherapy, and one patient has not yet completed chemotherapy. Of the 7 pts completing chemotherapy and proceeding to T-cell infusion, 5 pts achieved a CR/CRu and 2 pts achieved a PR; 5 pts received 1 – 5 x 109 CD3+ cells and 2 patients received 5 – 10 x 109 CD3+ cells. There have been no adverse events related to T-cell infusions. Median follow-up after ACTC infusion is 19 mos (range: 3 – 26 mos). CD4+ counts increased in all patients by 1 month after T-cell infusion, with a median increase of 3.2 fold (range: 1.5 – 70, p= .028)(see figure). For patients at dose level 1, the median increase was 2.2 fold (n=4; range: 1.5 – 3.3); at dose level 2 it was 37 fold (n=2; range: 3.8 – 70). CD8+ counts also increased, with a median increase of 8.1 (range: 1.0 – 30, p= .046)(see figure). All 7 pts receiving ACTC were anergic to candida antigen by delayed type hypersensitivity (DTH) skin testing before chemotherapy. Four pts developed a positive DTH response to candida antigen 60 days after ACTC infusion. For patients receiving ACTC, median follow-up is 24 mos (range: 7 – 31 mos) with a median progression-free survival of 18 months which is significantly longer than the time to progression from last therapy (p= .024).
CONCLUSIONS: ACTC infusion results in significant CD4+ and CD8+ numerical and functional lymphocyte recovery after cyclophosphamide-fludarabine chemotherapy in pts with low-grade FL. This compares very favorably to historical controls treated with fludarabine-based regimens. T-cell dose escalation is ongoing.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal