Abstract
Aims: MSCs are cells being investigated for use in various therapies including facilitation of HSC transplantation and as gene therapy delivery vehicles. We have explored the potential to increase the number of bone marrow (BM) MSCs in vivo, induce mobilization using various cytokine regimens and improve gene transfer into these cells with adeno-associated virus (AAV) in a baboon model.
Method: Baboons received cytokines as follows: 1. G-CSF 100mcg/kg/day for 5 days; 2. pegylated G-CSF (pegG-CSF), single dose 300mcg/kg day −5; 3. G-CSF 100mcg/kg/day + stem cell factor (SCF) 50mcg/kg/day for 5 days; and 4. pegylated megakaryocyte growth and development factor (pegMGDF) 1mcg/kg second daily for 10 days + G-CSF 100mcg/kg/day for 5 days starting day −5. Animals underwent BM aspiration at baseline and on the final day of cytokines along with leukapheresis to isolate PBMNCs for detection of peripheral blood (PB) CFU-F. The immunophenotype and differentiation potential of CFU-F derived from animals before and after cytokines was compared. The ability of AAV vectors pseudotyped with capsids derived from AAV of serotypes 1, 2, 3, 4, 5, 6, and 8 to mediate transduction of baboon and human MSCs was assessed.
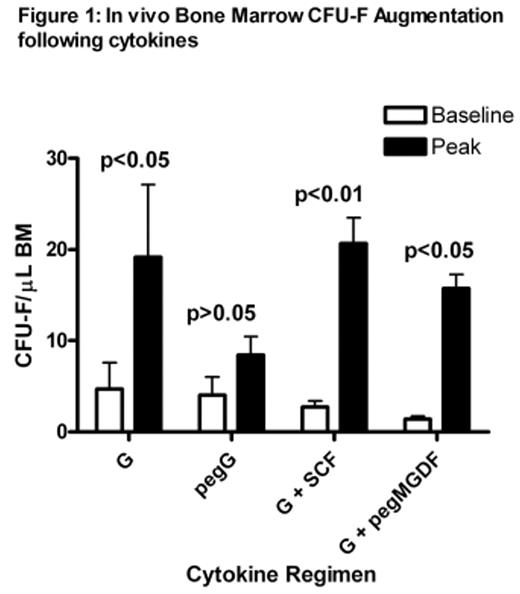
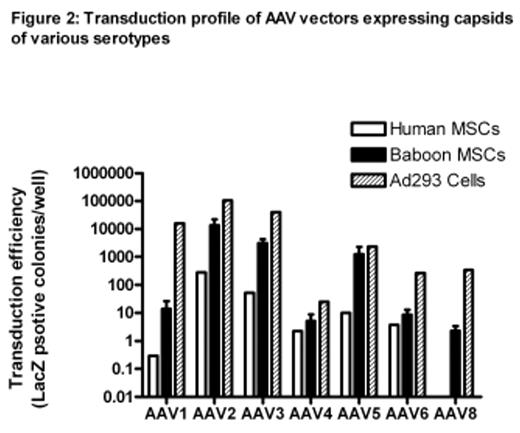
Results: Augmentation of bone marrow MSCs was observed with all cytokine regimens with the fold-increase compared to baseline as follows: 4.1, 2.1, 7.6 and 11.2 after G-CSF, pegG-CSF, G-CSF+SCF and G-CSF+pegMGDF respectively (see Figure 1). The immunophenotype of MSCs obtained after cytokines was identical to baseline cells as was their differentation potential. CFU-F were not detected in baseline PB however they were detected in 3/5 animals after G-CSF+SCF at a frequency of 0.8 to 1.5/mL, but no other cytokine regimen. A similar pattern of transduction efficiency using AAV was shared by human and baboon MSCs (see Figure 2) using control Ad293 cells. Specifically AAV vectors expressing capsids of serotypes 2, 3 and 5 were most efficient in transducing human and baboon MSCs. Those expressing capsids from serotypes 1, 4, 6, and 8 were much less efficient in transducing MSCs from either species. Baboon MSCs were able to be transduced by about 100-fold more than their human equivalent cells using AAV serotypes 2, 3, and 5.
Conclusion: This is the first report of mobilization of primate MSCs and, together with the demonstration of in vivo augmentation and AAV gene transfer, offers increased therapeutic opportunities for their safe application in a burgeoning number of diseases.
Transduction profile of AAV vectors expressing capsids of various serotypes
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal