Abstract
The efficacy of single agent arsenic trioxide (ATO) in the management of relapsed and newly diagnosed patients with acute promyelocytic leukaemia (APL) has been established. The impact of FLT3 activating mutations and additional cytogenetic changes in newly diagnosed patients with APL treated with single agent ATO has never been reported. Between January 1998 and April 2005, 98 newly diagnosed cases of PML-RARα positive APL were treated with a regimen of single agent ATO at our centre. FLT3 activating mutations were seen in 33% (FLT3-ITD - 21.3%, D835V- 11.7%). Of the 69 patients evaluated for additional cytogenetic findings, 76.8% had an isolated t(15;17) while 23.2% had t(15;17) along with additional cytogenetic findings.
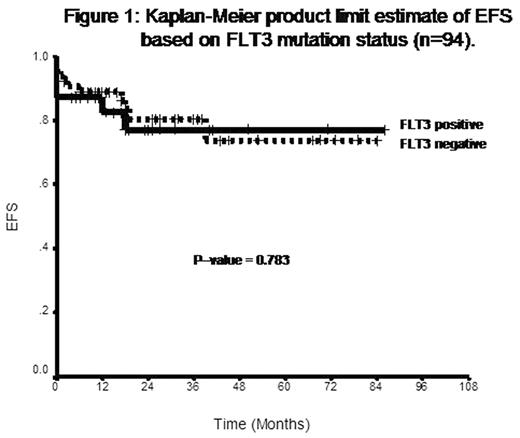
The presence of a FLT3 mutation was significantly associated with a higher mean white cell count (18.11±23.74 vs. 8.04±21.44, P=0.024) and a bcr3 PML-RARα isoform (48.4% vs. 20.6%, P=0.006). There was also a significant delay in achieving a molecular remission (MR) among patients with a FLT3 mutation as noted by the number of patients who were in MR prior to onset of consolidation therapy (60.9% vs. 85.7%, P=0.031). There was no significant difference in the hematological remission rate (CR), time to CR or early mortality between the group with and without a FLT3 mutation. At a median follow up of 20 months (range: 4 – 97), the 3 year Kaplan-Meier estimate of OS, EFS and DFS for patients with a FLT3 mutation was 82.51±7.24, 77.01±8.6, 88.82±7.48 percent while for those without a FLT3 mutation it was 92.06±3.4, 80.45±5.86, 89.02±5.31 percent. Statistical analysis of these survival curves by a log rank test did not reveal any significant difference between the two groups [Figure 1].
The presence of additional cytogenetic changes was not associated with any significant differences in the clinical or laboratory parameters at presentation. It also did not have an impact on the CR, MR, EFS, DFS or OS. Neither FLT3 activating mutations nor additional cytogenetic changes have a significant impact on the outcome of newly diagnosed patients with APL treated with single agent ATO in the short term. Whether either of these two parameters will affect long term outcome remains to be seen.
Kaplan-Meier product limit estimate of EFS based on FLT3 mutation status (n=94).
Kaplan-Meier product limit estimate of EFS based on FLT3 mutation status (n=94).
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal