Abstract
The nucleophosmin (NPM) - anaplastic lymphoma kinase (ALK) fusion protein, which is the product of the balanced chromosomal rearrangement t(2;5)(p23;q35), occurs in about 50% of nodal anaplastic large cell lymphoma (ALCL). Expression of this fusion kinase results in neoplastic transformation by modulating multiple intracellular signaling molecules, such as the PI3-kinase and the ERK1/2 kinases.
Here we show high activation of JunB in various human ALCL cell lines. Moreover, using human embryonic kidney (HEK293) and murine hematopoietic Ba/F3 cells ectopically expressing NPM-ALK, we demonstrate that NPM-ALK is the trigger for JunB activation.
Knock down of JUNB in NPM-ALK expressing cells using RNA interference results in upregulation of p16INK4a and downregulation of Cyclin D1, causing G1/S cell cycle arrest. Thus, JunB plays a critical oncogenic role in NPM-ALK transformed cells.
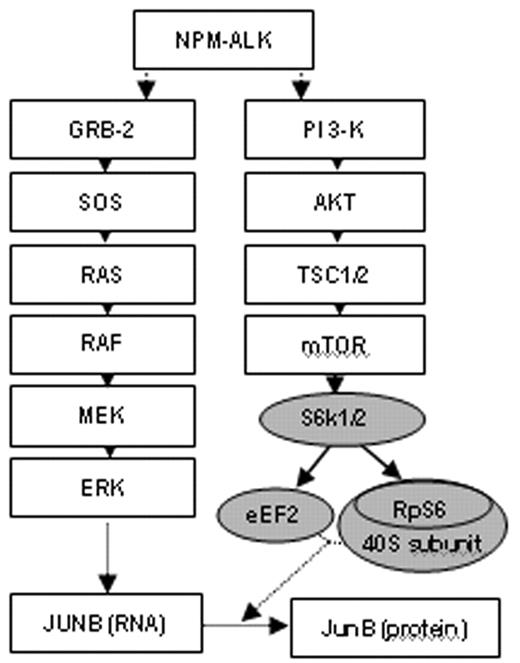
Using the PI3-kinase inhibitor LY294002 and the MEK-inhibitor UO126, we demonstrate that NPM-ALK-induced JunB activity as well as cellular proliferation are dependent on both PI3-kinase and MEK-ERK signaling. Moreover, we illustrate that NPM-ALK and subsequently PI3-kinase activate AKT and mTOR/S6K1 which are implicated in the translational control of specific mRNA molecules containing a polypyrimidine motif (see pathway diagram). Since JUNB mRNA harbors such a motif we confirmed that JUNB mRNA is translated in large polysomes using ribosomal gradient preparations. Selective block of mTOR with the immunosuppressant rapamycin decreases proliferation and reduces protein but not mRNA levels of JunB in human and murine NPM-ALK positive cell lines. A similar effect is seen when inhibiting the upstream activator of mTOR, PI3-kinase. In contrast, a significant decrease of JUNB mRNA levels is shown in cells treated by the MEK-inhibitor UO126.
An analogous effect of NPM-ALK is observed in ALCL patient samples. Hyperphosphorylation of S6K1 at Thr389 indicating activated mTOR/S6K1 is seen in 9/10 NPM-ALK positive ALCL cases in contrast to only 1/15 of NPM-ALK negative ALCL samples (P < 0,001). Hence, these findings suggest that the signaling cascade NPM-ALK→PI3K→AKT→mTOR/S6K1 is crucial to induce JUNB translation in human NPM-ALK positive ALCL.
In conclusion, we reveal that JunB acts as an oncogene in NPM-ALK positive neoplastic cells and therefore represents a valid therapeutic target. Our data indicate a novel mechanism for regulation of AP-1 activity in general and suggest a new therapeutic approach to specifically modulate translation of an oncogene.
Disclosures: FWF (Austrian Science Foundation).
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal