Abstract
Hematopoietic stem cells (HSC) can generate all hematopoietic cells. HSCs are defined by their pluripotency and their capacity for self-renewal; however, the factors that control these properties are poorly understood. The Wnt signaling pathway has been implicated in HSC self-renewal. It has been shown by Reya et al that the β-catenin-dependent Wnt pathway is involved in this process. Also, Wnt3a, when included in culture medium, has been shown to maintain HSC in their undifferentiated state. However, due to the redundant and interchangeable roles of Wnt molecules, the impacts of Wnt pathways in HSC-niche interaction and cell fate decisions are not well-understood. To identify the Wnt players in HSC cell fate decision, we have examined the expression of Wnt pathway components in HSCs and terminally differentiated cells including both the lymphoid (NK cells, T cells, and B cells) myeloid (granulocytes and macrophages), and erythroid lineages. Of 42 Wnt-pathway molecules examined at the mRNA level, we have discovered seven to be uniquely expressed in HSCs. Interestingly, most of these genes are Wnt receptors, including receptors of both canonical (Fzd4 and Fzd7) and non-canonical (Fzd3, Fzd6, and Ryk) Wnt pathways (Figure. 1). The non-canonical pathway has previously been found to exert distinct functions from the canonical pathway and is implicated in asymmetric cell divisions during development. Taken together with the ubiquitous expression patterns of the downstream molecules of both canonical and non-canonical pathways in all hematopoietic cells, we hypothesize that the canonical and non-canonical Wnt pathways have unique impacts on HSC cell fate decisions through the activation of Wnt receptors. In order to explore these roles, we generated retroviral vectors to mediate overexpression of each of the 7 pathway components that are uniquely expressed in HSC. These vectors have been used to transduce bone marrow cells, which were then transplanted into mice. RNAi knock-down vectors for each gene are also being generated. The impact of these gene expression modulations on HSC maintenance and differentiation is being determined by serial analysis of peripheral blood and bone marrow engraftment of transplant recipients.
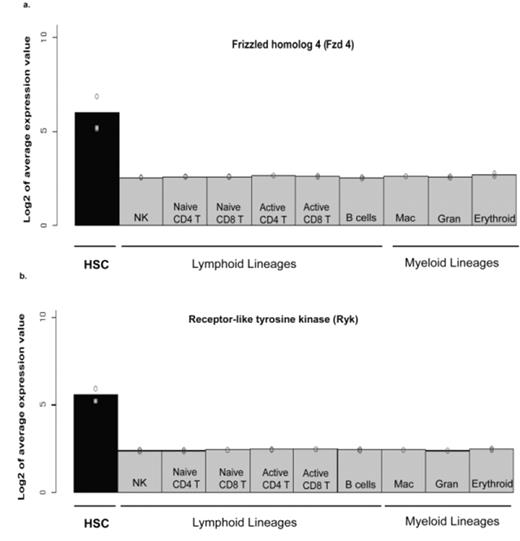
Expression of Wnt receptors in hematopoietic cells. Several Wnt receptors have been found to be uniquely expressed in HSC when compared to the terminally-differentiated lineages, including lymphoid cells (NK cells, T cells, and B cells) and myeloid cells (macrophages, granulocytes, and erythrocytes). Among them, both canonical receptors such as Fzd4 (a) and non-canonical receptors such as Ryk (b) are highly expressed in HSC. The average expression value was derived from a normalized microarray (AffyMoe430 2.0 Array) data (n=2).
Expression of Wnt receptors in hematopoietic cells. Several Wnt receptors have been found to be uniquely expressed in HSC when compared to the terminally-differentiated lineages, including lymphoid cells (NK cells, T cells, and B cells) and myeloid cells (macrophages, granulocytes, and erythrocytes). Among them, both canonical receptors such as Fzd4 (a) and non-canonical receptors such as Ryk (b) are highly expressed in HSC. The average expression value was derived from a normalized microarray (AffyMoe430 2.0 Array) data (n=2).
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal